
ORGANIC CHEMISTRY-ACCESS
6th Edition
ISBN: 9781260475586
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 40P
Give the IUPAC name for each compound.
a. 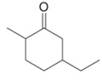 c.
c. 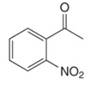 e.
e. 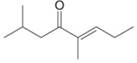
b. 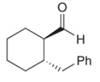 d.
d. 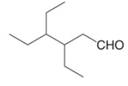 f.
f. 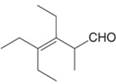
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the mechanism to make the alcohol 2-hexanol.
Draw the Mechanism to make the alcohol 1-hexanol.
Draw the mechanism for the formation of diol by starting with 1-pentanal in...
basic conditions
then
acidic conditions
then draw the mechanism for the formation of a carboxylic acid from your product.
Identify each chiral carbon as either R or S. Identify the overall carbohydrates as L or D
Chapter 18 Solutions
ORGANIC CHEMISTRY-ACCESS
Ch. 18.1 - Rank the following compounds in order of...Ch. 18.1 - Prob. 2PCh. 18.2 - Give the IUPAC name for each aldehyde.Ch. 18.2 - Prob. 4PCh. 18.2 - Give the IUPAC name for each ketone.Ch. 18.5 - Prob. 11PCh. 18.9 - Problem 21.17 Draw the products of the following...Ch. 18.9 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 18.9 - Problem 21.19 Draw the products (including...Ch. 18.9 - Problem 21.20 What starting materials are needed...
Ch. 18.9 - Prob. 19PCh. 18.10 - Problem 21.22 The product formed when reacts with...Ch. 18.10 - Prob. 21PCh. 18.11 - Prob. 22PCh. 18.11 - Prob. 23PCh. 18.11 - Prob. 24PCh. 18.12 - Prob. 25PCh. 18.12 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 18.13 - Problem 21.29 Draw the products of each...Ch. 18 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 18 - 21.41 Rank the following compounds in order of...Ch. 18 - Prob. 39PCh. 18 - 21.43 Give the IUPAC name for each compound.
a....Ch. 18 - 21.44 Give the structure corresponding to each...Ch. 18 - Prob. 42PCh. 18 - 21.46 Draw the products of each reaction.
a. e....Ch. 18 - Prob. 44PCh. 18 - 21.48 Draw all stereoisomers formed in each...Ch. 18 - Prob. 54PCh. 18 - Prob. 55PCh. 18 - Prob. 56PCh. 18 - Devise a synthesis of each alkene using a Wittig...Ch. 18 - Prob. 60PCh. 18 - Prob. 62PCh. 18 - Prob. 63PCh. 18 - 21.64 Draw a stepwise mechanism for the following...Ch. 18 - 21.65 Draw a stepwise mechanism f or the following...Ch. 18 - Prob. 67PCh. 18 - 21.67 Draw a stepwise mechanism for each...Ch. 18 - Prob. 69PCh. 18 - Prob. 70P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY