
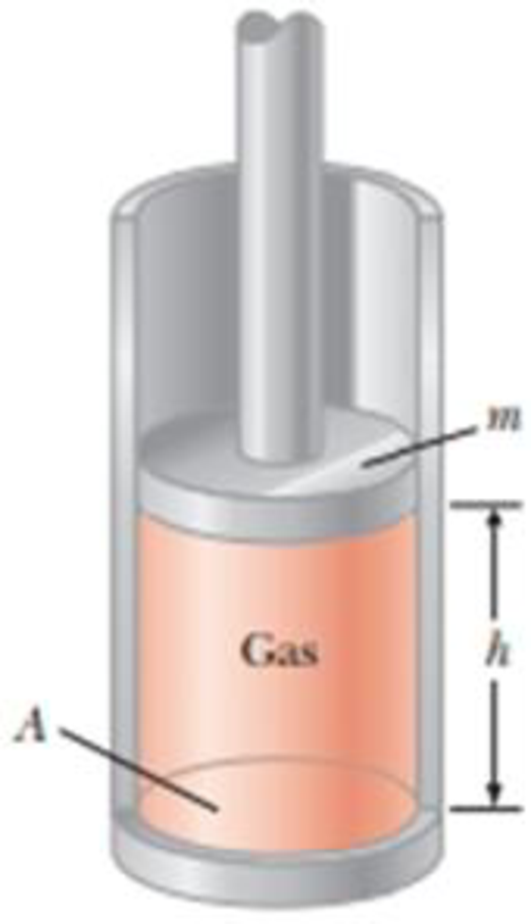
A vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P18.40). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find the height h in Figure P18.40. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder.
Figure P18.40
Trending nowThis is a popular solution!

Chapter 18 Solutions
Bundle: Physics For Scientists And Engineers With Modern Physics, Loose-leaf Version, 10th + Webassign Printed Access Card For Serway/jewett's Physics For Scientists And Engineers, 10th, Single-term
- PROBLEM 3 Cables A and B are Supporting a 185-lb wooden crate. What is the magnitude of the tension force in each cable? A 20° 35° 185 lbsarrow_forwardThe determined Wile E. Coyote is out once more to try to capture the elusive Road Runner of Loony Tunes fame. The coyote is strapped to a rocket, which provide a constant horizontal acceleration of 15.0 m/s2. The coyote starts off at rest 79.2 m from the edge of a cliff at the instant the roadrunner zips by in the direction of the cliff. If the roadrunner moves with constant speed, find the minimum velocity the roadrunner must have to reach the cliff before the coyote. (proper sig fig in answer)arrow_forwardPROBLEM 4 What is the resultant of the force system acting on the connection shown? 25 F₁ = 80 lbs IK 65° F2 = 60 lbsarrow_forward
- Three point-like charges in the attached image are placed at the corners of an equilateral triangle as shown in the figure. Each side of the triangle has a length of 38.0 cm, and the point (C) is located half way between q1 and q3 along the side. Find the magnitude of the electric field at point (C). Let q1 = −2.80 µC, q2 = −3.40 µC, and q3 = −4.50 µC. Thank you.arrow_forwardSTRUCTURES I Homework #1: Force Systems Name: TA: PROBLEM 1 Determine the horizontal and vertical components of the force in the cable shown. PROBLEM 2 The horizontal component of force F is 30 lb. What is the magnitude of force F? 6 10 4 4 F = 600lbs F = ?arrow_forwardThe determined Wile E. Coyote is out once more to try to capture the elusive Road Runner of Loony Tunes fame. The coyote is strapped to a rocket, which provide a constant horizontal acceleration of 15.0 m/s2. The coyote starts off at rest 79.2 m from the edge of a cliff at the instant the roadrunner zips by in the direction of the cliff. If the roadrunner moves with constant speed, find the minimum velocity the roadrunner must have to reach the cliff before the coyote. (proper sig fig)arrow_forward
- Hello, I need some help with calculations for a lab, it is Kinematics: Finding Acceleration Due to Gravity. Equations: s=s0+v0t+1/2at2 and a=gsinθ. The hypotenuse,r, is 100cm (given) and a height, y, is 3.5 cm (given). How do I find the Angle θ1? And, for distance traveled, s, would all be 100cm? For my first observations I recorded four trials in seconds: 1 - 2.13s, 2 - 2.60s, 3 - 2.08s, & 4 - 1.95s. This would all go in the coloumn for time right? How do I solve for the experimental approximation of the acceleration? Help with trial 1 would be great so I can use that as a model for the other trials. Thanks!arrow_forwardAfter the countdown at the beginning of a Mario Kart race, Bowser slams on the gas, taking off from rest. Bowser get up to a full speed of 25.5 m/s due to an acceleration of 10.4 m/s2. A)How much time does it take to reach full speed? B) How far does Bowser travel while accelerating?arrow_forwardThe drawing in the image attached shows an edge-on view of two planar surfaces that intersect and are mutually perpendicular. Side 1 has an area of 1.90 m^2, Side 2 has an area of 3.90 m^2, the electric field in magnitude is around 215 N/C. Please find the electric flux magnitude through side 1 and 2 combined if the angle (theta) made between the electric field with side 2 is 30.0 degrees. I believe side 1 is 60 degrees but could be wrong. Thank you.arrow_forward
- After the countdown at the beginning of a Mario Kart race, Bowser slams on the gas, taking off from rest. Bowser get up to a full speed of 25.5 m/s due to an acceleration of 10.4 m/s2.arrow_forwardThe drawing in the image attached shows an edge-on view of two planar surfaces that intersect and are mutually perpendicular. Side 1 has an area of 1.90 m^2, Side 2 has an area of 3.90 m^2, the electric field in magnitude is around 215 N/C. Please find the electric flux magnitude through side 1 and 2 combined if the angle (theta) made between the electric field with side 2 is 30.0 degrees. Thank you.arrow_forwardThe drawing in the image attached shows an edge-on view of two planar surfaces that intersect and are mutually perpendicular. Surface (1) has an area of 1.90 m^2, while Surface (2) has an area of 3.90 m^2. The electric field in magnitude of 215 N/C. Please find the magnitude of the electric flux through surface (with both 1 and 2 combined) if the angle (theta) made between the electric field with surface (2) is 30.0 degrees. Thank you.arrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





