
EBK ORGANIC CHEMISTRY
12th Edition
ISBN: 9781119233664
Author: Snyder
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 2PP
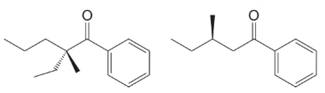
Practice Problem 18.2
Would optically active
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY
Ch. 18 - Prob. 1PPCh. 18 - Practice Problem 18.2 Would optically active...Ch. 18 - Prob. 3PPCh. 18 - Practice Problem 18.4 Why do we say that the...Ch. 18 - Prob. 5PPCh. 18 - Practice Problem 18.6 (a) Write a reaction...Ch. 18 - PRACTICE PROBLEM 18.7
Show how you would use the...Ch. 18 - Practice Problem 18.8 The acetoacetic ester...Ch. 18 - Practice Problem 18.9
In the synthesis of the keto...Ch. 18 - PRACTICE PROBLEM 18.10 How would you use the...
Ch. 18 - PRACTICE PROBLEM 18.11
How would you use the...Ch. 18 - PRACTICE PROBLEM 18.12 Outline all steps in a...Ch. 18 - PRACTICE PROBLEM 18.13
The antiepileptic drug...Ch. 18 - PRACTICE PROBLEM 18.14 Show how you could employ...Ch. 18 - Prob. 15PCh. 18 - Treating a solution of cis-1-decalone with base...Ch. 18 - Prob. 17PCh. 18 - Prob. 18PCh. 18 - Prob. 19PCh. 18 - Prob. 20PCh. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - The synthesis of cyclobutanecarboxylic acid given...Ch. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Compound J, a compound with two four-membered...Ch. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - 18.32 Shown below is a synthesis of the elm bark...Ch. 18 - 18.33 (a) A compound U gives a negative iodoform...Ch. 18 - 18.34 Compound A has the molecular formula and...Ch. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - 1. -Carotene is a highly conjugated hydrocarbon...Ch. 18 - Dehydroabietic acid is a natural product isolated...
Additional Science Textbook Solutions
Find more solutions based on key concepts
A water sample could be negative for Enterococcus and coliforms and still be a major public health threat. Why?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
1. Which parts of the skeleton belong to the appendicular skeleton? Which belong to the axial skeleton?
Human Anatomy & Physiology (2nd Edition)
During exponential growth, a population always (A) has a constant per capita population growth rate. (B) quickl...
Campbell Biology (11th Edition)
12.1 Give the IUPAC name for each of the following:
a. CH3-CH2-OH
b.
c.
d.
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
In Fig. 5-21, forces F1 and F2 are applied to a lunchbox as it slides at constant velocity over a frictionless ...
Fundamentals of Physics Extended
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License