
To find:
a) Sketch the unit cell of
b) Calculate the density of
c) Calculate the percent empty space in a unit cell of
Answer to Problem 18.93QA
Solution:
a)
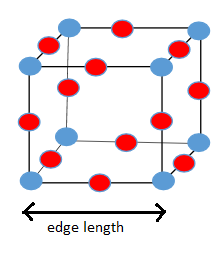
b)
c)
Explanation of Solution
1) Concept:
a) Unit cell of

b)
c) The packing efficiency is calculated using volumes of one
2) Formula:
i)
ii)
iii)
iv)
3) Given:
i)
ii)
4) Calculations:
b)
Volume of a unit cell:
Molar mass of
Mass of a unit cell of
Volume of
Volume of
So total volume of a
So,
This is the space occupied by
Conclusion:
From the diagram of unit cell of
Want to see more full solutions like this?
Chapter 18 Solutions
CHEM:ATOM FOC 2E CL (TEXT)
- Find a mole of : H2SO4 (15 ml 3.0 M) NaOH ( 18 mL 2.5 M) Acetic anhydride (3.0 mL)arrow_forwardFor the mechanism, show everything, lone paires, charges and arrow pleasearrow_forwardmolecule 0= OH ☐ ☐ type of molecule (check all that apply) fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated ☐ polyunsaturated ☐ ☐ ☐ ☐ ☐ 010 0 0 0 0 0 0 ☐ ☐ ☐ ☐☐☐☐ U omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 OH OHarrow_forward
- '☐ : ☑ ด Suppose an alien life form has DNA just like human DNA remain the same.) - except that the alien DNA is made from deoxyarabinose instead of deoxyribose. (All other ingredients Draw the structure of a nucleotide containing thymine from which the alien DNA would be assembled. Note: be sure to draw the molecule as it would exist at physiological pH. Click and drag to start drawing a structure.arrow_forwardPredict the products of the following biochemical reaction: CH2 CH-O + 3 KOH CH2-0 In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the No reaction box under the drawing area. Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw the complete right-hand side of the equation, including stoichiometric coefficients. No reaction Click and drag to start drawing a structure. : 5 èarrow_forwardAssign these spectrumarrow_forward
- If I have 30% H2O2, indicate how to prepare a 6% H2O2 solution.arrow_forward7) 8) FCI II -C-C-C=C-C || Br Br || -C=C-Br -CEC-C-C- 10) 11) F Br i OH مله 12) Br i 13) 14) 15) CH3CHFCHFC=CH C(OH)Br2CHF(CH2)4CH2CH3 CH3(CH2)3CH=CH(CH2)2CH3arrow_forwardName 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





