
(a)
Interpretation:
The reactivity of cyclohexanol and phenol with aqueous NaOH solution is to be stated.
Concept introduction:
The phenol is an aromatic compound having a formula, C6H5−OH in which carbon has sp2 hybridization. On the other hand, cyclohexanol is a compound having a six-member cyclic ring attached to −OH group in which carbon has sp2 hybridization. The reaction of an aqueous solution of NaOH which is a stronger base with alcohol or phenol generally forms sodium salt by abstracting the acidic proton.
Answer to Problem 18.53AP
Phenol is more reactive than cyclohexanol with an aqueous NaOH solution. Due to more stability of the conjugate base of phenol as compared to cyclohexanol.
Explanation of Solution
The sodium hydroxide is a strong base. It abstracts a proton from the stronger acidic compound and forms its sodium salt. In the given case, sodium hydroxide abstracts a proton from phenol and cyclohexanol and form phenoxide ion and cyclohexanol conjugate base respectively. The phenoxide ion gets stabilized by resonance structures, but this is not possible in case of cyclohexanol conjugate base. It is known that more the stability of the conjugate base more the acidity of the compound. In the given case, phenoxide ion is more stable due to this phenoxide is more acidic. Therefore, phenoxide ion is more reactive toward an aqueous NaOH solution. The resonance structure of the phenoxide ion is shown below.
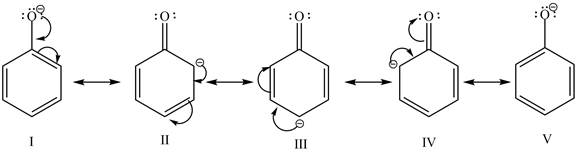
Figure 1
The reactivity of phenol is more as compared to cyclohexanol with an aqueous solution of NaOH. It is due to the more stability of the conjugate base of phenol.
(b)
Interpretation:
The reactivity of cyclohexanol and phenol with NaH in THF is to be stated.
Concept introduction:
The phenol is an aromatic compound having a formula, C6H5−OH in which carbon has sp2 hybridization. On the other hand, cyclohexanol is a compound having a six-member cyclic ring attached to −OH group in which carbon has sp2 hybridization. The reaction of an aqueous solution of NaH which is a stronger base with alcohol or phenol generally forms sodium salt by abstracting the acidic proton and hydrogen gas is evolved.
Answer to Problem 18.53AP
Phenol is more reactive than cyclohexanol with an aqueous NaH solution. Due to more stability of the conjugate base of phenol as compared to cyclohexanol.
Explanation of Solution
The sodium hydride is a strong base. It abstracts a proton from the stronger acidic compound and forms its sodium salt and hydrogen gas is evolved. In the given case, sodium hydroxide abstracts a proton from phenol and cyclohexanol and form phenoxide ion and cyclohexanol conjugate base respectively. The phenoxide ion gets stabilized by resonance structures, but this is not possible in case of cyclohexanol conjugate base. It is known that more the stability of the conjugate base more the acidity of the compound. In the given case, phenoxide ion is more stable due to this phenoxide is more acidic. Therefore, phenoxide ion is more reactive toward an aqueous NaH solution. The resonance structure of the phenoxide ion is shown below.
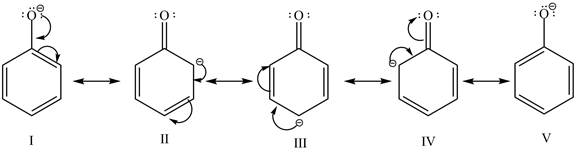
Figure 2
The reactivity of phenol is more as compared to cyclohexanol with an aqueous solution of NaH. It is due to the more stability of the conjugate base of phenol.
(c)
Interpretation:
The reactivity of cyclohexanol and phenol with triflic anhydride in pyridine at 0 °C is to be stated.
Concept introduction:
The triflic anhydride is a chemical compound which is also known as Trifluoromethanesulfonic anhydride. It has a molecular formula (CF3SO2)2O. It acts as a strong electrophile. It is derived from strong acid that is triflic acid. It reacts readily with nucleophiles.
Answer to Problem 18.53AP
The presence of the more nucleophilic character of cyclohexanol makes it more reactive towards triflic anhydride in pyridine at 0 °C as compared to phenol.
Explanation of Solution
In the given case, when triflic acid reacts with cyclohexanol and phenol in the presence of pyridine at 0 °C, it forms an ester as a product. The electron density of cyclohexanol is more due to the presence of +I effect of carbon which makes it better nucleophile. It is due to the participation of oxygen lone pair of electrons in resonance structure which decreases its nucleophilic activity. Therefore, triflic acid reacts easily with cyclohexanol in the presence of pyridine at 0 °C. The resonance structure of phenol is shown below.
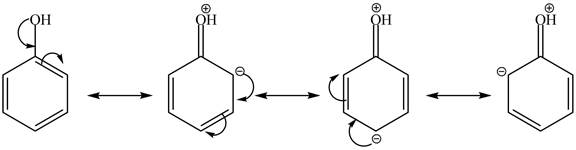
Figure 3
The reactivity of cyclohexanol is more as compared to phenol with triflic acid in pyridine at 0 °C. It is due to the more nucleophilic character of cyclohexanol.
(d)
Interpretation:
The reactivity of cyclohexanol and phenol with concentrated aqueous HBr, H2SO4 in presence of catalyst is to be stated.
Concept introduction:
The phenol is an aromatic compound having a formula, C6H5−OH in which carbon has sp2 hybridization. On the other hand, cyclohexanol is a compound having a six-member cyclic ring attached to −OH group in which carbon has sp2 hybridization. The saturated compound undergoes addition reaction whereas aromatic compound undergoes substitution reaction.
Answer to Problem 18.53AP
The deactivation of the aromatic ring of phenol makes it less reactive toward concentrated aqueous HBr, H2SO4, catalyst.
Explanation of Solution
Cyclohexanol is more nucleophilic as compared to phenol. It is due to the participation of the lone pair of electrons on oxygen in resonance structures of phenol. Therefore, in the given conditions cyclohexanol reacts more rapidly as compared to phenol. When cyclohexanol undergoes protonation in the presence of H2SO4 and catalyst followed by β-elimination, it results in the formation of cyclohexene. It further undergoes an addition reaction with HBr which results in the formation of bromocyclohexane. The reaction is shown below.
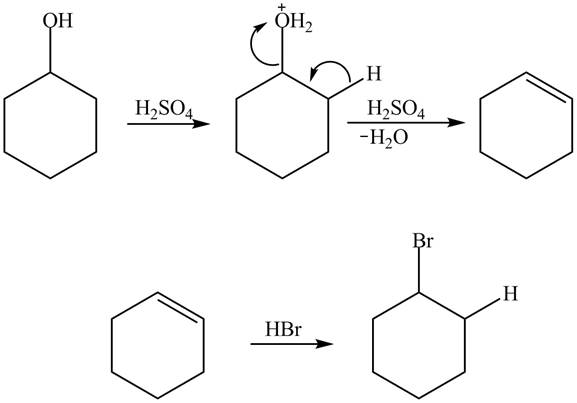
Figure 4
In the case of phenol, it undergoes electrophilic aromatic substitution reaction with HBr, but the reaction rate is slower as compared to cyclohexanol. It is due to protonation of the hydroxyl group of phenol which deactivates the aromatic ring toward electrophilic substitution. The generation of bromide ion also becomes difficult due to the presence of strong acid H2SO4. Therefore, cyclohexanol is more reactive toward concentrated aqueous HBr . H2SO4, catalyst.
The reactivity of phenol is less with concentrated aqueous HBr, H2SO4 and catalyst. Due to protonation of the hydroxyl group of phenol which deactivates the aromatic ring toward electrophilic substitution.
(e)
Interpretation:
The reactivity of cyclohexanol and phenol with Br2 in CCl4 . dark is to be stated.
Concept introduction:
The phenol is an aromatic compound having a formula, C6H5−OH in which carbon has sp2 hybridization. On the other hand, cyclohexanol is a compound having a six-member cyclic ring attached to −OH group in which carbon has sp2 hybridization. The saturated compound undergoes addition reaction whereas aromatic compound undergoes substitution reaction.
Answer to Problem 18.53AP
The phenol is more reactive toward Br2 in CCl4, dark because it is an aromatic compound which undergoes electrophilic substitution reaction.
Explanation of Solution
The phenol is an aromatic compound which shows similar reactions as
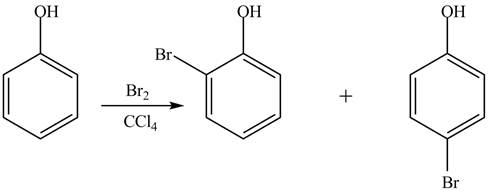
Figure 5
Whereas no such reaction is possible with cyclohexanol because this compound gives addition reaction.
The phenol is an aromatic compound which undergoes electrophilic substitution reaction. Due to this, it is more reactive toward Br2 in CCl4, dark.
(f)
Interpretation:
The reactivity of cyclohexanol and phenol with Na2Cr2O7, in H2SO4 is to be stated.
Concept introduction:
Oxidation is defined as the addition of oxygen atom or removal of the hydrogen atom. The oxidizing agent is the substance that causes oxidation and itself get reduced. The reagent Na2Cr2O7, in H2SO4 acts as a strong acidic oxidizing agent. Phenol on oxidation gives different products as expected from alcohols like cyclohexanol.
Answer to Problem 18.53AP
The phenol is an aromatic compound which loses its aromatic character on reaction with Na2Cr2O7, in H2SO4 that is why it is less reactive as compared to cyclohexanol.
Explanation of Solution
In the given conditions, both the given compounds undergo an oxidation reaction. The reaction of cyclohexanol with Na2Cr2O7, in H2SO4 gives cyclohexanone. The reaction is shown below.
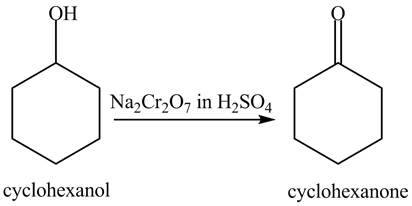
Figure 6
The reaction of phenol with Na2Cr2O7, in H2SO4 result in the formation of p−quinone. The reaction is shown below.
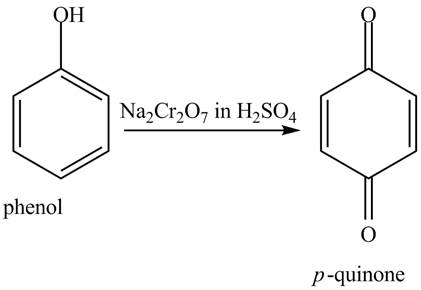
Figure 7
When phenol undergoes oxidation reaction, it loses its aromatic character which means it loses its stability. Therefore, cyclohexanol is more reactive toward Na2Cr2O7, in H2SO4.
The phenol on reaction with Na2Cr2O7, in H2SO4 loses its aromatic character which decreases its reactivity towards Na2Cr2O7, in H2SO4.
(g)
Interpretation:
The reactivity of cyclohexanol and phenol with H2SO4, heat is to be stated.
Concept introduction:
The phenol is an aromatic compound having a formula, C6H5−OH in which carbon has sp2 hybridization. On the other hand, cyclohexanol is a compound having a six-member cyclic ring attached to −OH group in which carbon has sp2 hybridization.
Answer to Problem 18.53AP
The deactivation of the aromatic ring of phenol due to protonation of the hydroxyl group makes it less reactive toward H2SO4, heat.
Explanation of Solution
Cyclohexanol is more nucleophilic as compared to phenol. It is due to the participation of the lone pair of electrons on oxygen in resonance structures of phenol. Therefore, in the given conditions cyclohexanol reacts more rapidly as compared to phenol. When cyclohexanol undergoes protonation in the presence of H2SO4, catalyst followed by β elimination, it results in the formation of cyclohexene. The reaction is shown below.
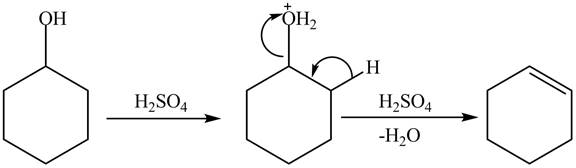
Figure 8
The less nucleophilic character makes phenol less reactive toward H2SO4, heat.
The reactivity of phenol is less with H2SO4, heat. Due to protonation of the hydroxyl group of phenol which deactivates the aromatic ring.
Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- I need help with the followingarrow_forwardI need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forward
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forwardI need help with the follloaingarrow_forwardFor a CARS experiment on a Raman band 918 cm-1, if omega1= 1280 nm, calculate the omega2 in wavelength (nm) and the CARS output in wavelength (nm).arrow_forward
- I need help with the following questionarrow_forwardFor CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

