
Concept explainers
The diagram here shows an electrolytic cell consisting of a Co electrode in a 2.0 M Co(NO3)2 solution and a Mg electrode in a 2.0 M Mg(NO3)2 solution. (a) Label the anode and cathode and show the half-cell reactions. Also label the signs (+ or −) on the battery terminals. (b) What is the minimum voltage to drive the reaction? (c) After the passage of 10.0 A for 2.00 h the battery is replaced with a voltmeter and the electrolytic cell now becomes a galvanic cell. Calculate Ecell. Assume volumes to remain constant at 1.00 L in each compartment.
a)
Interpretation:
Half reactions; the anode and cathode have to be labelled.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
Thermodynamics of redox reactions:
The change in free-energy represents the maximum amount of useful work that can be obtained in a reaction:
Relation between
Relation between
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a redox reaction under nonstandard-state conditions is,
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
Explanation of Solution
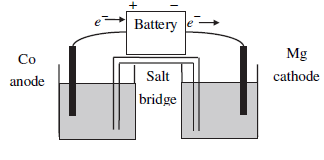
Figure.1
For the given redox reactions,
In the galvanic cell, Oxidation occurs at anode and reduction occurs at cathode.
Therefore,
Anode (Oxidation):
Cathode (Reduction):
b)
Interpretation:
The minimum voltage needed to drive the reaction has to be calculated.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
Explanation of Solution
The emf values for the two given half-reactions are,
Anode (Oxidation):
Cathode (Reduction):
Calculated standard emf for galvanic cell as follows,
The minimum voltage needed to drive the reaction is
c)
Interpretation:
The emf of a given galvanic cell has to be calculated.
Concept introduction:
Standard reduction potential: The voltage associated with a reduction reaction at an electrode when all solutes are 1M and all gases are at 1 atm. The hydrogen electrode is called the standard hydrogen electrode (SHE).
Standard emf:
Where both
Effect of concentration on cell Emf:
The mathematical relationship between the emf of galvanic cell and the concentration of reactants and products in a redox reaction under nonstandard-state conditions is,
As known
Dividing by –nF, the above equation becomes,
Nernst equation: The Nernst equation is used to calculate the cell voltage under nonstandard-state conditions.
Explanation of Solution
Given: Current= 10.0 A; Time,
Convert Current into coulomb:
As known,
Convert number of Coulombs into mole of electrons:
Convert mole of electrons into number of moles:
1 mole of Cobalt
‘X’of Cobalt =
Therefore, no.of moles of Cobalt is
Assuming solution volumes of 1.00L, the concentration of Co2+ in solution after 2hours is 2.373 M, and the concentration of Mg2+ in solution after 2 hours is 1.627 M. we use the Nernst equation to solve for
Calculation of non-standard emf value using Nernst equation:
The reaction quotient for the given reaction is,
The concentration of pure solids and pure liquids do not appear in the expression for Q.
Hence, the reaction quotient becomes,
Substitute known constant values of R, T and F into Nernst equation becomes as follows,
The number of electrons transferred in the given redox reaction is TWO (n=2) and
The emf of the given cell reaction is
Want to see more full solutions like this?
Chapter 18 Solutions
Chemistry
- 3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). CN + En CNarrow_forward3) Propagation of uncertainty. Every measurement has uncertainty. In this problem, we'll evaluate the uncertainty in every step of a titration of potassium hydrogen phthalate (a common acid used in titrations, abbreviated KHP, formula CsH5KO4) with NaOH of an unknown concentration. The calculation that ultimately needs to be carried out is: concentration NaOH 1000 x mass KHP × purity KHP molar mass KHP x volume NaOH Measurements: a) You use a balance to weigh 0.3992 g of KHP. The uncertainty is ±0.15 mg (0.00015 g). b) You use a buret to slowly add NaOH to the KHP until it reaches the endpoint. It takes 18.73 mL of NaOH. The uncertainty of the burst is 0.03 mL.. c) The manufacturer states the purity of KHP is 100%±0.05%. d) Even though we don't think much about them, molar masses have uncertainty as well. The uncertainty comes from the distribution of isotopes, rather than random measurement error. The uncertainty in the elements composing KHP are: a. Carbon: b. Hydrogen: ±0.0008…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- How would you use infrared spectroscopy to distinguish between the following pairs of constitutional isomers? (a) CH3C=CCH3 || and CH3CH2C=CH (b) CH3CCH=CHCH3 and CH3CCH2CH=CH2 Problem 12-41 The mass spectrum (a) and the infrared spectrum (b) of an unknown hydrocarbon are shown. Propose as many structures as you can. (a) 100 Relative abundance (%) 80 60 60 40 200 20 (b) 100 Transmittance (%) 10 20 20 80- 60- 40- 20 40 60 80 100 120 140 m/z 500 4000 3500 3000 2500 2000 1500 Wavenumber (cm-1) 1000arrow_forwardPropagation of uncertainty. You have a stock solution certified by the manufacturer to contain 150.0±0.03 µg SO42-/mL. You would like to dilute it by a factor of 100 to obtain 1.500 µg/mL. Calculate the uncertainty in the two methods of dilution below. Use the following uncertainty values for glassware: Glassware Uncertainty (assume glassware has been calibrated and treat the values below as random error) 1.00 mL volumetric pipet 0.01 mL 10.00 mL volumetric pipet 0.02 mL 100.00 mL volumetric flask 0.08 mL Transfer 10.00 mL with a volumetric pipet and dilute it to 100 mL with a volumetric flask. Then take 10.00 mL of the resulting solution and dilute it a second time with a 100 mL flask. 2. Transfer 1.00 mL with a volumetric pipet and dilute it to 100 mL with a volumetric flask.arrow_forwardDraw all resonance structures for the following ion: CH₂ Draw all resonance structures on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single bond is active by default. 2D ד CONT HD EXP CON ? 1 [1] Α 12 Marvin JS by Chemaxon A DOO H C N Br I UZ OSPFarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





