
(a)
Interpretation:
A
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an
Answer to Problem 11ST
Alpha emission of
Explanation of Solution
Alpha particle emission releases one alpha particle whose mass number decreases by
![]()
Figure 1
Radioactive alpha decay of
(b)
Interpretation:
A nuclear equation for the decay of
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an atomic number greater than
Answer to Problem 11ST
Beta emission of
Explanation of Solution
Beta emission decays into a proton and releases an electron or the atomic number increases by
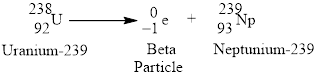
Figure 2
Radioactive beta decay of
(c)
Interpretation:
A nuclear equation for the decay of
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an atomic number greater than
Answer to Problem 11ST
Positron emission of
Explanation of Solution
In positron emission, an electron is released and its charge is
![]()
Figure 3
Radioactive positron electron emission of
(d)
Interpretation:
A nuclear equation for the decay of
Concept introduction:
Radioactive decay is the process that involves the emission of radiation by an unstable atomic nucleus. The atomic nucleus loses its energy. The process is spontaneous. It is also known as nuclear radiation. The decay is accompanied by the emission of alpha particles, beta particles, and gamma rays and so on. All the elements which have an atomic number greater than
Answer to Problem 11ST
Electron capture of
Explanation of Solution
In electron capture decay, element accepts electron and results in atomic number decreased by 1 and mass number remains same. The electron capture of
![]()
Figure 4
Radioactive alpha decay of
Want to see more full solutions like this?
Chapter 18 Solutions
EBK INTRODUCTORY CHEMISTRY
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





