
Concept explainers
What carbonyl compounds might you start with to prepare the Following compounds by Grignard reaction? List all possibilities.
(a) 2-Methyl-2-propanol
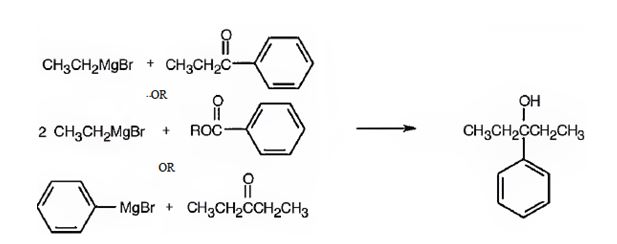
(b) 1-Ethylcyclohexanol
(c) 3-Phenyl-3-pentanol
(d) 2-Phenyl-2-pentanol
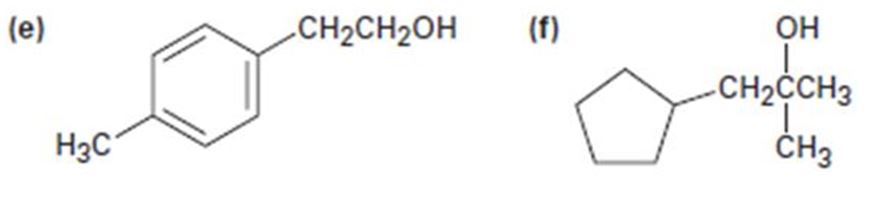
a) 2-Methyl-2-propanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-methyl-2-propanol are to be listed.
Concept introduction:
Grignard reagents react with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-methyl-2-propanol.
Answer to Problem 44AP
2-methyl-2-propanol is a 30 alcohol. It can be prepared by treating acetone with methylmagnesium bromide or an acetic ester with two molar equivalents of methylmagnesium bromide.
Explanation of Solution
A four carbon 30 alcohol is required. Hence a three carbon ketone (acetone) is treated with methylmagnesium bromide. In the case of esters two carbons will be provided by methylmagnesium bromide since esters require two molar equivalents of the reagent. Hence an ester of the two carbon acid (acetic acid) is chosen.
2-methyl-2-propanol is a 30 alcohol. It can be prepared by treating acetone with methylmagnesium bromide or an acetic ester with two molar equivalents of methylmagnesium bromide.
b) 1-Ethylcyclohexanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-ethylcyclohexanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-ethylcyclohexanol.
Answer to Problem 44AP
1-Ethylcyclohexanol can be prepared by treating cyclohexanone with ethylmagnesium bromide.
Explanation of Solution
A six-membered cyclic 30 alcohol with ethyl group on C1 is required. Hence a six membered cyclic ketone (cyclohexanone) is treated with a two carbon Grignard reagent (ethylmagnesium bromide).
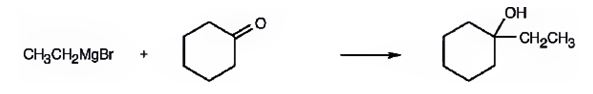
1-Ethylcyclohexanol can be prepared by treating cyclohexanone with ethylmagnesium bromide.
c) 3-Phenyl-3-pentanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 3-phenyl-3-pentanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 3-phenyl-3-pentanol.
Answer to Problem 44AP
3-Phenyl-3-pentanol can be prepared by reacting i) ethylphenyl ketone with ethylmagnesium bromide ii) benzoic acid esters with two molar equivalents of ethylmagnesium bromide iii) diethyl ketone with phenylmagnesium bromide.
Explanation of Solution
3-Phenyl-3-pentanol is a 30 alcohol with a five carbon straight chain with a –OH and phenyl groups on C3. Hence an aromatic ketone (ethylphenyl ketone) is treated with ethylmagnesium bromide or the ester of benzoic acid is treated with two equivalents of ethylmagnesium bromide. The ring can come from the Grignard reagent also. Hence phenylmagnesium bromide is treated with diethyl ketone.
3-Phenyl-3-pentanol can be prepared by reacting i) ethylphenyl ketone with ethylmagnesium bromide ii) benzoic acid esters with two molar equivalents of ethylmagnesium bromide iii) diethyl ketone with phenylmagnesium bromide.
d) 2-Phenyl-2-pentanol
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-phenyl-2-pentanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-phenyl-2-pentanol.
Answer to Problem 44AP
2-phenyl-2-pentanol can be prepared by reacting i) methylphenyl ketone with propylmagnesium bromide ii) phenylpropyl ketone with methylmagnesium bromide iii) methylpropyl ketone with phenylmagnesium bromide.
Explanation of Solution
2-Phenyl-2-pentanol is a 30 alcohol with a five carbon straight chain with a –OH and phenyl groups on C2. Hence an aromatic ketone like methylphenyl ketone is treated with propylmagnesium bromide or phenylpropyl ketone is treated methylmagnesium bromide. The ring can come from the Grignard reagent also. Hence phenylmagnesium bromide is treated with methylpropyl ketone.
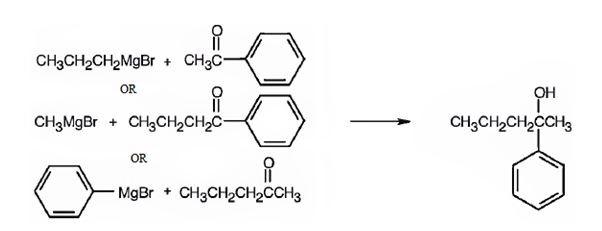
2-phenyl-2-pentanol can be prepared by reacting i) methylphenyl ketone with propylmagnesium bromide ii) phenylpropyl ketone with methylmagnesium bromide iii) methylpropyl ketone with phenylmagnesium bromide.
e)
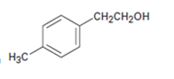
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-p-tolylethanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 2-(p-tolyl) ethanol.
Answer to Problem 44AP
2-(p-tolyl) ethanol can be prepared by reacting formaldehyde with p-tolylmethylmagnesium bromide.
Explanation of Solution
2-(p-tolyl) ethanol is a 10 alcohol having a p-tolyl group attached to C2 of ethanol. Hence formaldehyde is required. The remaining part should come from the Grignard reagent. Hence formaldehyde is treated with p-tolylmethylmagnesium bromide.
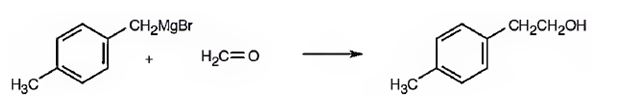
2-(p-tolyl) ethanol can prepared by reacting formaldehyde with p-tolylmethylmagnesium bromide.
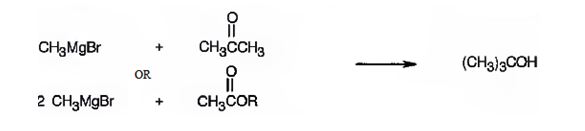
f)
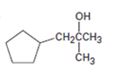
Interpretation:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-cyclopentyl-2-methyl-2-propanol are to be listed.
Concept introduction:
Grignard reagents react with formaldehyde to produce 10 alcohols, with other aldehydes to yield 20 alcohols and with ketones to give 30 alcohols as the product. Esters also when treated with two molar equivalents of Grignard reagents yield 30 alcohols.
To list:
All possible carbonyl compounds that will react in a Grignard reaction to yield 1-cyclopentyl-2-methyl-2-propanol are to be listed.
Answer to Problem 44AP
1-Cyclopentyl-2-methyl-2-propanol can be prepared by reacting i) cyclopentylmethyl methyl ketone with methylmagnesium bromide ii) an ester of cyclopentylacetic acid with two molar equivalents of methylmagnesium bromide iii) acetone with cyclopentylmethylmagnesium bromide.
Explanation of Solution
1-Cyclopentyl-2-methyl-2-propanol is a 30 alcohol with a three carbon straight chain with a cyclopentyl group on C1 and –OH on C2. Hence cyclopentylmethyl methyl ketone is treated with methylmagnesium bromide or an ester of cyclopentylacetic acid is treated with two molar equivalents of methylmagnesium bromide. The ring can come from the Grignard reagent also. Hence cyclopentylmethylmagnesium bromide is treated with acetone.
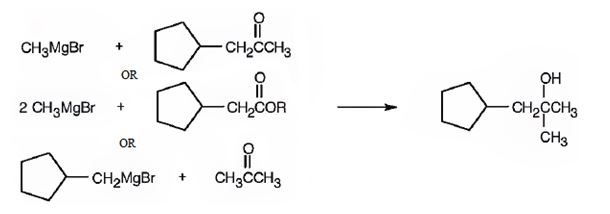
1-Cyclopentyl-2-methyl-2-propanol can be prepared by reacting i) cyclopentylmethyl methyl ketone with methylmagnesium bromide ii) an ester of cyclopentylacetic acid with two molar equivalents of methylmagnesium bromide iii) acetone with cyclopentylmethylmagnesium bromide.
Want to see more full solutions like this?
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forward
- If the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forwardGiven the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forwardIndicate the functions that salt bridges have in batteries.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

