
(a)
Interpretation:
The given reaction is
The reaction quotient,
Concept Introduction:
Reaction quotient:
The ratio of concentration terms for a reaction is known as reaction quotient. It is denoted by
(a)
Explanation of Solution
The given reaction is
The reaction quotient for the reaction can be represented as
(b)
Interpretation:
The given filmstrip contains five molecular scenes of a gaseous mixture as it reaches equilibrium over time. In the filmstrip,
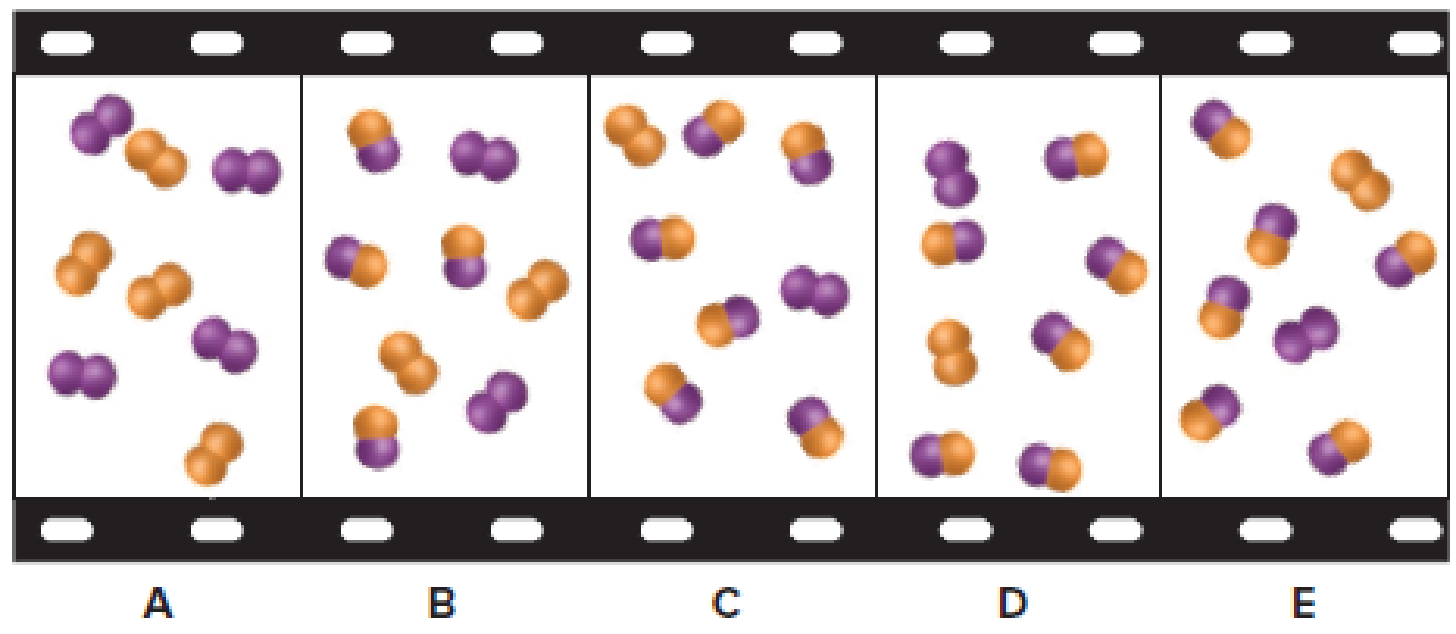
Figure 1
If each particle represents
Concept Introduction:
Reaction quotient:
The ratio of concentration terms for a reaction is known as reaction quotient. It is denoted by
(b)
Explanation of Solution
Scene A:
The
Scene B:
The
Scene C, D, E:
The
(c)
Interpretation:
The given reaction is
If
Concept Introduction:
At equilibrium,
If
If
If
(c)
Explanation of Solution
The given condition is
Scene-A:
The value of
Scene-B:
The value of
Scene-C, D, E:
The value of
As the value of
(d)
Interpretation:
The given reaction is
The value of
Concept Introduction:
Reaction quotient:
The ratio of concentration terms for a reaction is known as reaction quotient. It is denoted by
At equilibrium,
(d)
Explanation of Solution
As
(e)
Interpretation:
The given filmstrip contains five molecular scenes of a gaseous mixture as it reaches equilibrium over time. In the filmstrip,
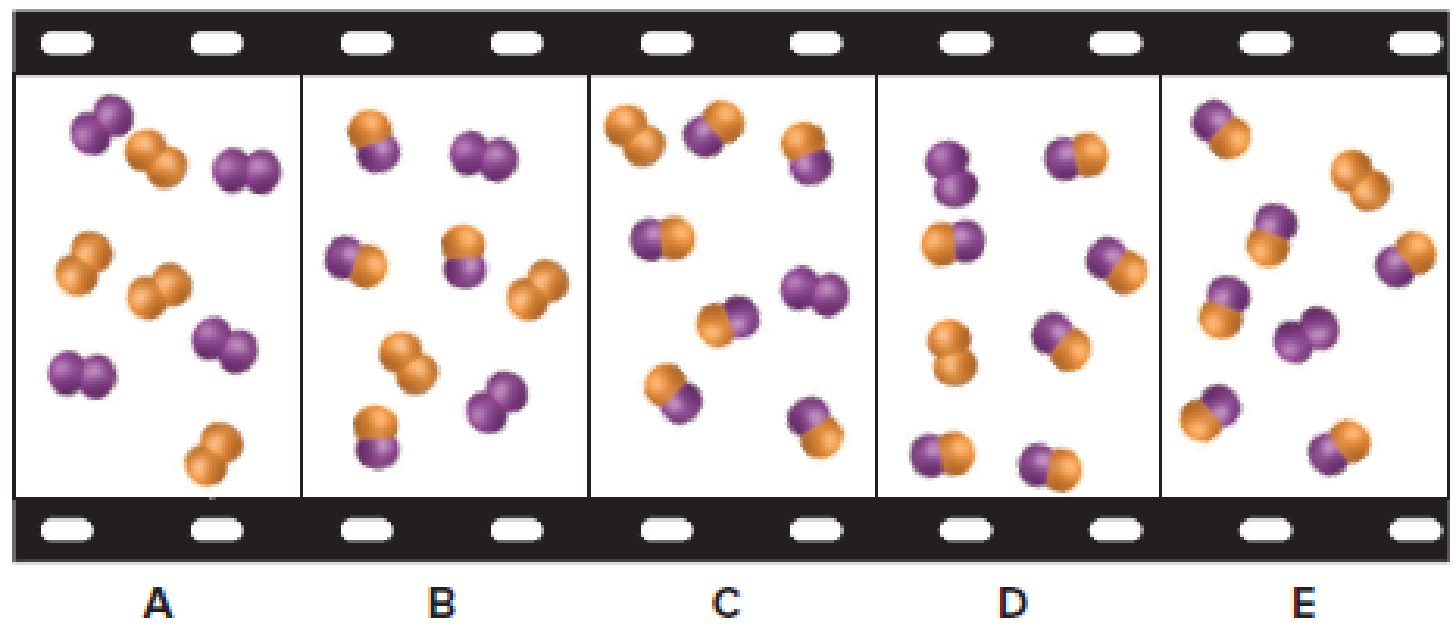
Figure 1
If
Concept Introduction:
Change in equilibrium due to temperature changes:
If the temperature is increased for the system, the equilibrium shifts away from the heat because of the reaction needs extra heat to use.
If the temperature is decreased for the system, the equilibrium shifts towards the heat because the heat needs to be produced to make up for the loss.
(e)
Explanation of Solution
Among the given five scenes, the scene that represents the mixture at a higher temperature is scene B. At higher temperatures, reaction shifts towards left forming more
(f)
Interpretation:
The given filmstrip contains five molecular scenes of a gaseous mixture as it reaches equilibrium over time. In the filmstrip,
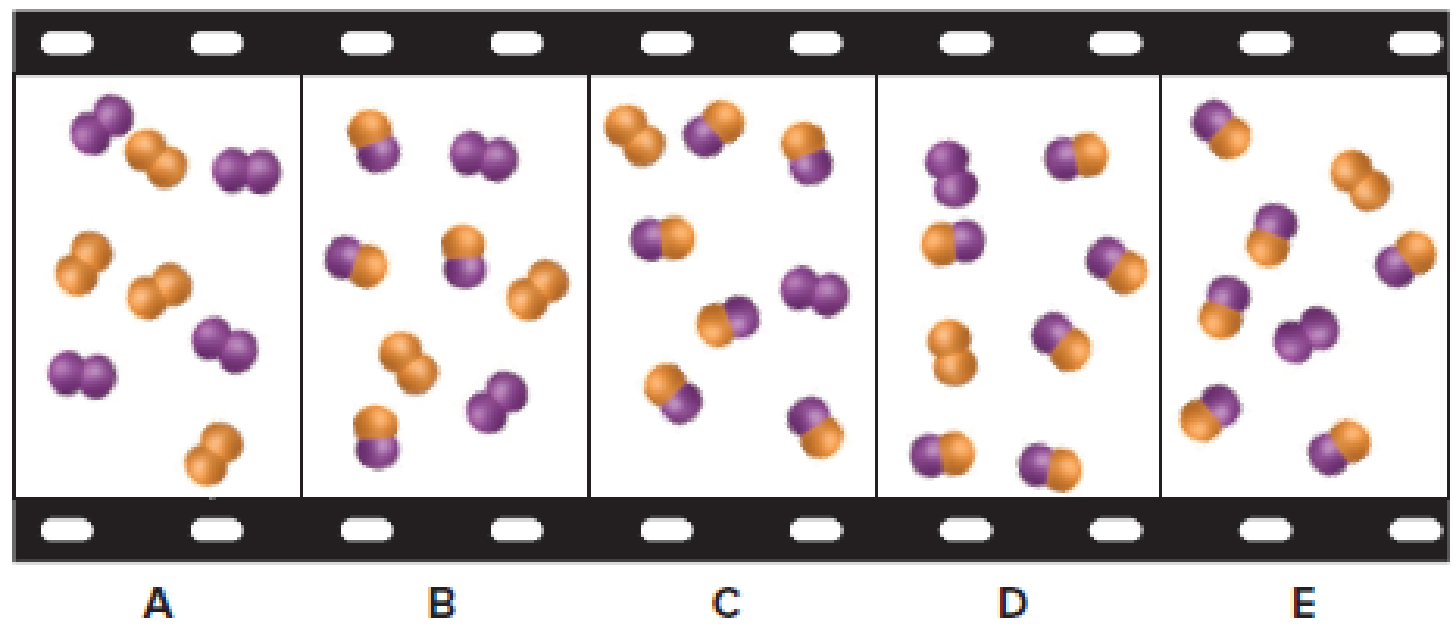
Figure 1
The scene that represents the mixture at a higher pressure (lower volume) has to be explained.
Concept Introduction:
Change in equilibrium due to pressure changes:
On increase in the system pressure, the equilibrium shifts towards fewer moles of gas, because, for gases,
On decrease in the system pressure, the equilibrium shifts towards more moles of gas, because, for gases,
(f)
Explanation of Solution
The given reaction is
The gaseous molecules in reactants are
Want to see more full solutions like this?
Chapter 17 Solutions
CHEMISTRY(LOOSELEAF) W/CONNECT+EBOOK
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
- In the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forwardCalculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





