
Concept explainers
(a)
Interpretation:
The synthesis of the given
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is shown below:
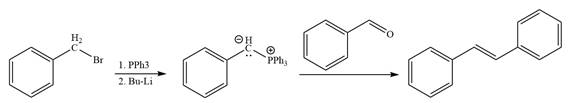
Explanation of Solution
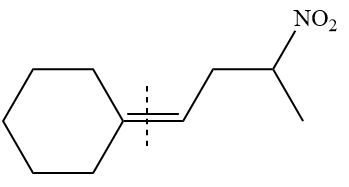
The given structure of alkene is
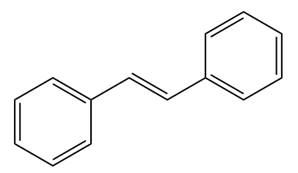
In the above structure, the
By undoing a Wittig reaction in retrosynthetic analysis,
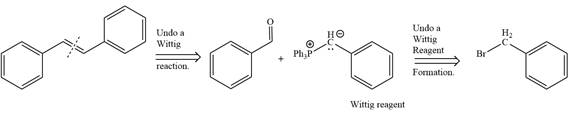
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehydewould appears as follows:
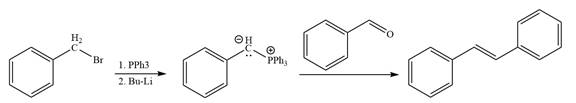
The synthesis of given alkene from an alkyl halide and a ketone or aldehyde is shown.
(b)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
Method 2:
Explanation of Solution
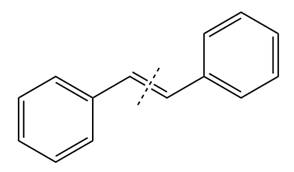
The given structure of alkene is
In the above structure, the
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
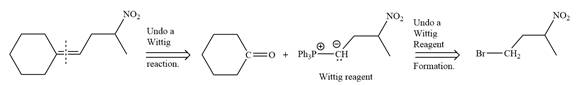
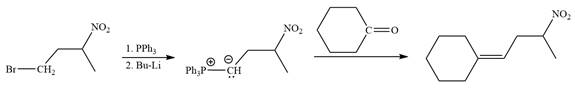
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:
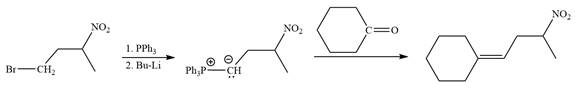
Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
In the forward direction, the synthesis of the given alkene from an alkyl halide and an aldehyde would appear as follows:

The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
(c)
Interpretation:
The synthesis of the given alkene from an alkyl halide and a ketone or aldehyde is to be shown.
Concept introduction:
Wittig reactions generate the carbon double(
Answer to Problem 17.56P
The synthesis of the givenunsymmetricalkene from an alkyl halide and a ketone or aldehyde by two different ways as shown below.
Method 1:
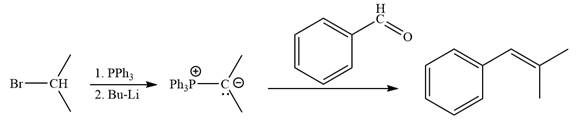
Method 2:
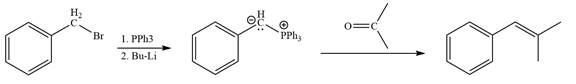
Explanation of Solution
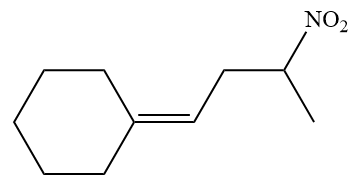
The given structure of alkene is
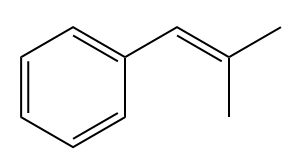
In the above structure, the
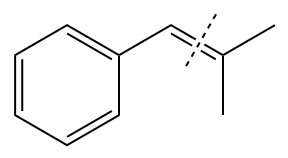
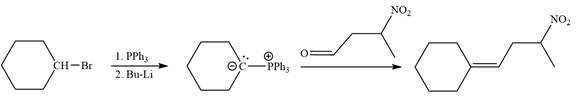
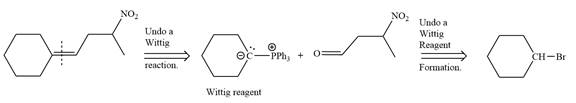
Method 1:
By undoing a Wittig reaction in retrosynthetic analysis,
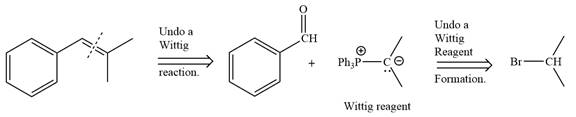
In the forward direction, the synthesis of the given alkene from an alkyl halide and benzaldehyde would appear as follows:

Method 2:
By undoing a Wittig reaction in retrosynthetic analysis,
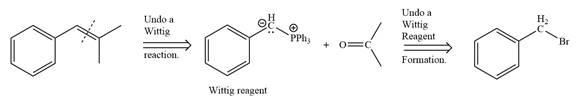
In the forward direction, the synthesis of the given alkene from an alkyl halide and a ketone would appear as follows:

The synthesis of the given unsymmetric alkene from an alkyl halide and a ketone or an aldehyde by two different ways is shown.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry: Principles And Mechanisms (second Edition)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
