
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 17.28UKC
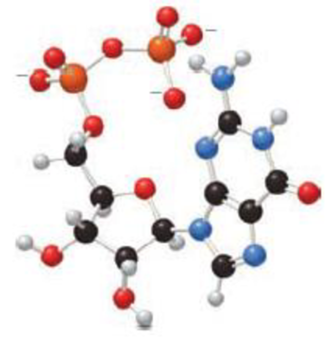
(a) Identify the base and monosaccharide in the following
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4-
yl)methanone
Ph
N-H
Ph
Draw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total
stereoisomers are there? Name the sugar you drew.
Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total
stereoisomers are there? Draw the enantiomer.
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Chapter 17 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 17.1 - Prob. 17.1PCh. 17.1 - Draw the structure of guanosine. Classify the...Ch. 17.1 - Prob. 17.3PCh. 17.1 - Give the name that corresponds to each...Ch. 17.1 - Which nucleic acid (DNA or RNA) contains each of...Ch. 17.1 - Label each statement about the compound...Ch. 17.1 - Identify each component as a base, nucleoside, or...Ch. 17.2 - Draw the structure of a dinucleotide formed by...Ch. 17.2 - Label the 5' end and the 3' end in each...Ch. 17.2 - Label each statement about the polynucleotide...
Ch. 17.3 - Write the complementary strand for each of the...Ch. 17.4 - What is the sequence of a newly synthesized DNA...Ch. 17.6 - Prob. 17.13PCh. 17.6 - Prob. 17.14PCh. 17.11 - Prob. 17.24PCh. 17 - Label each statement as pertaining to DNA, RNA, or...Ch. 17 - (a) Identify the base and monosaccharide in the...Ch. 17 - (a) Identify the base and monosaccharide in the...Ch. 17 - Consider the given dinucleotide. a. Identify the...Ch. 17 - Answer Problem 17.29 for the following...Ch. 17 - Fill in the codon, anticodon, or amino acid needed...Ch. 17 - Fill in the codon, anticodon, or amino acid needed...Ch. 17 - What is the difference between a gene and a...Ch. 17 - Prob. 17.38APCh. 17 - List three structural differences between DNA and...Ch. 17 - List three structural similarities in DNA and RNA.Ch. 17 - Prob. 17.41APCh. 17 - Prob. 17.42APCh. 17 - Give the name, abbreviation, or structure of each...Ch. 17 - Give the name, abbreviation, or structure of each...Ch. 17 - Classify each molecule as a nucleoside or...Ch. 17 - Draw the structure of the deoxyribonucleotide...Ch. 17 - Draw the structure of the ribonucleotide formed by...Ch. 17 - Describe in detail the DNA double helix with...Ch. 17 - Describe in detail the DNA double helix with...Ch. 17 - Write the sequence of the complementary strand of...Ch. 17 - Write the sequence of the complementary strand of...Ch. 17 - What is the sequence of the mRNA molecule...Ch. 17 - What is the sequence of the mRNA molecule...Ch. 17 - Considering each nucleotide sequence in an mRNA...Ch. 17 - Considering each nucleotide sequence in an mRNA...Ch. 17 - What is the difference between a point mutation...Ch. 17 - Consider the following mRNA sequence: CUU CAG CAC....Ch. 17 - Consider the following mRNA sequence: ACC UUA CGA....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following molecules are NOT typical carbohydrates? For the molecules that are carbohydrates, label them as an aldose or ketose. HO Он ОН ОН Он ОН но ΤΗ HO ОН HO eve Он он ОН ОН ОН If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units are present?arrow_forwardDraw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forwardName the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forward
- What are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forward
- Condensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forward
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY