
Concept explainers
Interpretation:
The reason behind the fact that 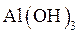 will precipitate and
will precipitate and 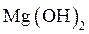 will not precipitate in qualitative analysis should be identified.
will not precipitate in qualitative analysis should be identified.
Concept introduction:
Solubility product is equilibrium constant for reactions that occur when an ionic compound is dissolved to produce ions. It is denotedby 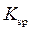 Consider
Consider 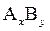 an ionic compound. Its dissociation occurs as:
an ionic compound. Its dissociation occurs as:
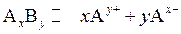
The expression for its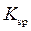 is as follows:
is as follows:
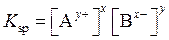
A precipitate of an ionic compound will form when solution that contains constituent ion is mixed. The precipitation depends on value of ion product (IP). The IP is defined in the same way as 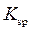 Also, the concentrations in expression for IP are concentration at time t and not equilibrium concentrations. Consider
Also, the concentrations in expression for IP are concentration at time t and not equilibrium concentrations. Consider 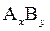 to be an ionic compound. Its dissociation occurs as follows:
to be an ionic compound. Its dissociation occurs as follows:
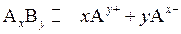
The expression for its IP is as follows:
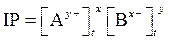
The negative logarithm of molar concentration of hydronium ion is called 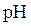 The expression for pH is as follows:
The expression for pH is as follows:
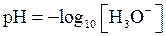
The relation between 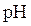 and
and 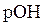 is as follows:
is as follows:
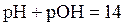
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- H 14. Draw the line angle form of the following molecule make sure you use the proper notation to indicate spatial positioning of atoms. F F H 15. Convert the following condensed form to line angle form: (CH3)3CCH2COCH2CON(CH2CH3)2arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both only if(A) the stoichiometry A:B is 1:1.(B) the stoichiometry A:B is 1:2 or 2:1.arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both.(1) Only if the stoichiometry A:B is 1:1.(2) If the initial quantities of A and B are in their stoichiometric ratios.arrow_forward
- There are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Calculate the measurement of uncertainty and provide the data in a spreadsheet table. Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32 152.87 151.24 153.26 152.02 152.90 152.87 151.49 152.46 152.58arrow_forward1. Predict the organic product(s) of the following reactions. Assume excess of reagents unless otherwise noted. a) &l BH3 •THF b) 1) NaOH 2) H3O+ solve d) ala 1) EtMgBr 2) H3O+ e) H2N سكر CuLi NH2 1) SOCI2 2) EtMgBr 3) H3O+ NC H3O+ Δarrow_forwardThere are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Summarise and report these results including an indication of measurement uncertainty. In both calculation samples calculate if an outlier is present, max value, number of samples, mean, standard deviation, g (suspect), g (critical) and t (critical). Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32…arrow_forward
- Indicate the rate expressions for reactions that have order 0, 1, and 2.arrow_forwardPROBLEMS Q1) Label the following salts as either acidic, basic, or neutral a) Fe(NOx) c) AlBr b) NH.CH COO d) HCOON (1/2 mark each) e) Fes f) NaBr Q2) What is the pH of a 0.0750 M solution of sulphuric acid?arrow_forward8. Draw all the resonance forms for each of the fling molecules or ions, and indicate the major contributor in each case, or if they are equivalent (45) (2) -PH2 سمة مدarrow_forward
- A J то گای ه +0 Also calculate the amount of starting materials chlorobenzaldehyde and p-chloroacetophenone required to prepare 400 mg of the given chalcone product 1, 3-bis(4-chlorophenyl)prop-2-en-1-one molar mass ok 1,3-bis(4-Chlorophenyl) prop-2-en-1-one = 277.1591m01 number of moles= 0.400/277.15 = 0.00144 moles 2 x 0.00 144=0.00288 moves arams of acetophenone = 0.00144 X 120.16 = 0.1739 0.1739x2=0.3469 grams of benzaldehyde = 0.00144X106.12=0.1539 0.1539x2 = 0.3069 Starting materials: 0.3469 Ox acetophenone, 0.3069 of benzaldehyde 3arrow_forward1. Answer the questions about the following reaction: (a) Draw in the arrows that can be used make this reaction occur and draw in the product of substitution in this reaction. Be sure to include any relevant stereochemistry in the product structure. + SK F Br + (b) In which solvent would this reaction proceed the fastest (Circle one) Methanol Acetone (c) Imagine that you are working for a chemical company and it was your job to perform a similar reaction to the one above, with the exception of the S atom in this reaction being replaced by an O atom. During the reaction, you observe the formation of three separate molecules instead of the single molecule obtained above. What is the likeliest other products that are formed? Draw them in the box provided.arrow_forward3. For the reactions below, draw the arrows corresponding to the transformations and draw in the boxes the reactants or products as indicated. Note: Part A should have arrows drawn going from the reactants to the middle structure and the arrows on the middle structure that would yield the final structure. For part B, you will need to draw in the reactant before being able to draw the arrows corresponding to product formation. A. B. Rearrangement ΘΗarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





