
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
12th Edition
ISBN: 9781337915984
Author: Bettelheim
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 15P
18-18 Propanoic acid and methyl acetate are constitutional isomers, and both are liquids at room temperature. One of these compounds has a boiling point of 141°C; the other has a boiling point of 57°C. Which compound has which boiling point? Explain.
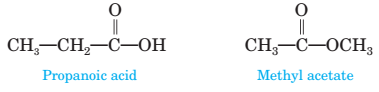
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can someone help me whats the issue?
a. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression:
AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T
A. Calculate the value of AS for the process.
B. Next, use the Gibbs-Helmholtz equation:
(a(AG/T))
ΔΗ
-
T2
to calculate the value of AH for the process.
None
Chapter 17 Solutions
EP INTRO.TO GENERAL,ORGANIC...-OWL ACCE
Ch. 17.2 - Prob. 17.1QCCh. 17.5 - Prob. 17.2QCCh. 17.5 - Prob. 17.3QCCh. 17 - 18-4 Answer true or false. (a) The functional...Ch. 17 - Prob. 2PCh. 17 - 18-6 Name and draw structural formulas for the...Ch. 17 - 18-7 Write the IUPAC name for each carboxylic...Ch. 17 - 18-8 Write the IUPAC name for each carboxylic...Ch. 17 - Prob. 6PCh. 17 - Prob. 7P
Ch. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10PCh. 17 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 17 - 18-15 Draw a structural formula for the dimer...Ch. 17 - 18-16 Propanedioic (malonic) acid forms an...Ch. 17 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 17 - 18-18 Propanoic acid and methyl acetate are...Ch. 17 - 18-19 The following compounds have approximately...Ch. 17 - Prob. 17PCh. 17 - Prob. 18PCh. 17 - Prob. 19PCh. 17 - 18-23 Characterize the structural features...Ch. 17 - Prob. 21PCh. 17 - Prob. 22PCh. 17 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 17 - Prob. 24PCh. 17 - 18-28 Arrange these compounds in order of...Ch. 17 - 18-29 Complete the equations for these acid—base...Ch. 17 - 18-30 Complete the equations for these acid-base...Ch. 17 - 18-31 Formic acid is one of the components...Ch. 17 - Prob. 29PCh. 17 - Prob. 30PCh. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Prob. 33PCh. 17 - Prob. 34PCh. 17 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 17 - Prob. 36PCh. 17 - Prob. 37PCh. 17 - 18-41 Complete these examples of Fischer...Ch. 17 - Prob. 39PCh. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Prob. 42PCh. 17 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 17 - 18-47 Methylparaben and propylparaben are used as...Ch. 17 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 17 - Prob. 46PCh. 17 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ASP please....arrow_forwardNonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forward
- Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forward
- Based on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forwardDraw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY