
Essential University Physics
4th Edition
ISBN: 9780134988559
Author: Wolfson, Richard
Publisher: Pearson Education,
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 16.3, Problem 16.3GI
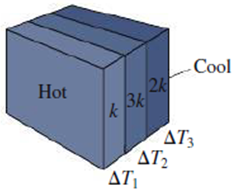
The figure shows three slabs with the same thickness but different thermal
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
need answer asap please thanks you
A man slides two boxes up a slope. The two boxes A and B have a mass of 75 kg and 50
kg, respectively.
(a) Draw the free body diagram (FBD) of the two crates.
(b) Determine the tension in the cable that the man must exert to cause imminent
movement from rest of the two boxes.
Static friction coefficient
USA = 0.25
HSB = 0.35
Kinetic friction coefficient
HkA = 0.20
HkB = 0.25
M₁ = 75 kg
MB = 50 kg
P
35°
Figure 3
B
200
A golf ball is struck with a velocity of 20 m/s at point A as shown below (Figure 4).
(a) Determine the distance "d" and the time of flight from A to B;
(b) Determine the magnitude and the direction of the speed at which the ball strikes the
ground at B.
10°
V₁ = 20m/s
35º
Figure 4
d
B
Chapter 16 Solutions
Essential University Physics
Ch. 16.1 - Is there (a) no temperature, (b) one temperature,...Ch. 16.2 - A hot rock with mass 250 g is dropped into an...Ch. 16.3 - The figure shows three slabs with the same...Ch. 16.3 - Prob. 16.4GICh. 16.4 - A houses thermostat fails, leaving the furnace...Ch. 16 - Does a thermometer measure its own temperature or...Ch. 16 - Compare the relative sizes of the kelvin, the...Ch. 16 - If you put a thermometer in direct sunlight, what...Ch. 16 - Why does the temperature in a stone building...Ch. 16 - Why do large bodies of water exert a...
Ch. 16 - Stainless-steel cookware often has a layer of...Ch. 16 - Prob. 7FTDCh. 16 - Glass and fiberglass are made from the same...Ch. 16 - To keep your hands warm while skiing, you should...Ch. 16 - Global warming at Earths surface is generally...Ch. 16 - Prob. 11ECh. 16 - A Canadian meteorologist predicts an overnight low...Ch. 16 - Normal room temperature is 68F. Whats this in...Ch. 16 - Prob. 14ECh. 16 - At what temperature do the Fahrenheit and Celsius...Ch. 16 - The normal boiling point of nitrogen is 77.3 K....Ch. 16 - Prob. 17ECh. 16 - Prob. 18ECh. 16 - Prob. 19ECh. 16 - Whats the specific heat of a material if it takes...Ch. 16 - The average human diet contains about 2000 kcal...Ch. 16 - Prob. 22ECh. 16 - Prob. 23ECh. 16 - Building heat loss in the United States is usually...Ch. 16 - Find the heat-loss rate through a slab of (a) wood...Ch. 16 - Youre a builder whos advising a homeowner to have...Ch. 16 - An 8.0 m by 12 m house is built on a concrete slab...Ch. 16 - Find the -factor for a wall that loses 0.040 Btu...Ch. 16 - Compute the -factors for 1-inch thicknesses of...Ch. 16 - A horseshoe has surface area 50 cm2, and a...Ch. 16 - An oven loses energy at the rate of 14 W per C...Ch. 16 - Youre having your homes heating system replaced,...Ch. 16 - The filament of a 100-W lightbulb is at 3.0 kK....Ch. 16 - A typical human body has surface area 1.4 nr and...Ch. 16 - Example 16.2: An iron frying pan of mass 2.65 kg...Ch. 16 - Prob. 36ECh. 16 - Example 16.2: During the refueling of a nuclear...Ch. 16 - Prob. 38ECh. 16 - Example 16.7: A solar greenhouse has 435 ft2 of...Ch. 16 - Prob. 40ECh. 16 - Example 16.7: An asteroid in the belt between Mars...Ch. 16 - A constant-volume gas thermometer is filled with...Ch. 16 - A constant-volume gas thermometer is at 55-kPa...Ch. 16 - In Fig. 16.2s gas thermometer, the height h is...Ch. 16 - Prob. 46PCh. 16 - Typical fats contain about 9 kcal per gram. If the...Ch. 16 - A circular lake 1.0 km in diameter is 10 m deep...Ch. 16 - How much heat is required to raise an 800-g copper...Ch. 16 - Initially, 100 g of water and 100 g of another...Ch. 16 - Prob. 51PCh. 16 - Two neighbors return from Florida to find their...Ch. 16 - Prob. 53PCh. 16 - In the 2011 nuclear accident at Fukushima, Japan,...Ch. 16 - Prob. 55PCh. 16 - The temperature of the eardrum provides a reliable...Ch. 16 - Prob. 57PCh. 16 - Prob. 58PCh. 16 - A piece of copper at 300C is dropped into 1.0 kg...Ch. 16 - While camping, you boil water to make spaghetti....Ch. 16 - A biology labs walk-in cooler measures 3.0 m by...Ch. 16 - One end of an iron rod 40 cm long and 3.0 cm in...Ch. 16 - Prob. 63PCh. 16 - An electric stove burner has surface area 325 cm2...Ch. 16 - Youre considering purchasing a new sleeping bag...Ch. 16 - A blacksmith heats a 1.1-kg iron horseshoe to...Ch. 16 - Whats the power output of a microwave oven that...Ch. 16 - A cylindrical log 15 cm in diameter and 65 cm long...Ch. 16 - A blue giant star whose surface temperature is 23...Ch. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Estimate the average temperature on Pluto,...Ch. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - In a cylindrical pipe where area isnt constant....Ch. 16 - Prob. 79PCh. 16 - Prob. 80PCh. 16 - A passive solar house has south-facing windows...Ch. 16 - A more realistic approach to the solar greenhouse...Ch. 16 - Fiberglass is a popular, economical, and fairly...Ch. 16 - Fiberglass is a popular, economical, and fairly...Ch. 16 - Fiberglass is a popular, economical, and fairly...Ch. 16 - Fiberglass is a popular, economical, and fairly...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. The leading hypothesis for the origin ...
Cosmic Perspective Fundamentals
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
What is the mass of 225 mL of a liquid that has a density of 0.880 g/mL? a. 198 g b. 0.0039 g c. 0.198 g d. 0.2...
Introductory Chemistry (6th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The rectangular loop of wire shown in the figure (Figure 1) has a mass of 0.18 g per centimeter of length and is pivoted about side ab on a frictionless axis. The current in the wire is 8.5 A in the direction shown. Find the magnitude of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane. Find the direction of the magnetic field parallel to the y-axis that will cause the loop to swing up until its plane makes an angle of 30.0 ∘ with the yz-plane.arrow_forwardA particle with a charge of − 5.20 nC is moving in a uniform magnetic field of (B→=−( 1.22 T )k^. The magnetic force on the particle is measured to be (F→=−( 3.50×10−7 N )i^+( 7.60×10−7 N )j^. Calculate the y and z component of the velocity of the particle.arrow_forwardneed answer asap please thank youarrow_forward
- 3. a. Determine the potential difference between points A and B. b. Why does point A have a higher potential energy? Q = +1.0 C 3.2 cm 4.8 cm Aarrow_forwardPls help ASAParrow_forward1. Explain the difference between electrical field, potential difference, and electrical potential differencearrow_forward
- Pls help ASAParrow_forward9. When an electron moves into a uniform and perpendicular magnetic field, it will.. a. Accelerate parallel to the magnetic Field until it leaves b. Accelerate in a circular path c. Accelerate perpendicular to both the magnetic field and its original direction d. Repel back into the electric field 10. If a proton at rest is placed in a uniform magnetic field with no electric or gravitational field around, the proton will…….. a. Accelerate in the direction of the magnetic field b. Accelerate in a direction perpendicular to the magnetic field c. Move in a circular path d. Not acceleratearrow_forward7. The electric field at a distance of 1.0 mfrom a charged sphere is 100 N/C. At what distance from thesphere will the electric field be 50 N/C? a. 1.1 m b. 1.4 m c. 2.0 m d. 4.0 m 8. The electric potential due to a point charge at a point depends on a. The direction of the electric field b. The distance from the point charge c. The velocity of the point charge d. The mass of the point chargearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY