
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
14th Edition
ISBN: 9780136873891
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 7E
Which of these statements about how the percent ionization of a weak acid depends on acid concentration is true? [Section 16.6]
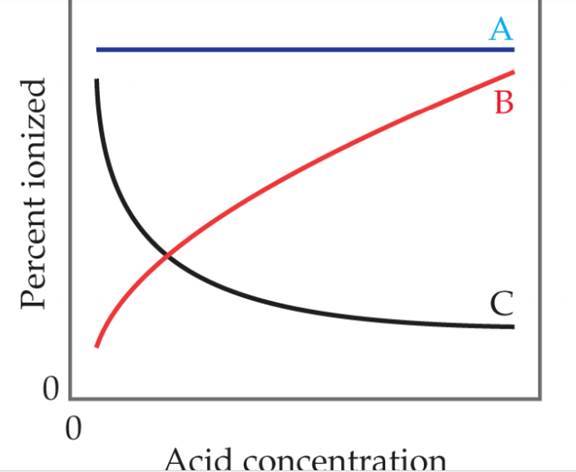
- Line A is most accurate because Ka does not depend on concentration.
- Line A is the most accurate because the percent ionization of the acid does not depend on concentration.
- Line B is the most accurate because as the acid concentration increases, a greater proportion of it is ionized.
- Line B is the most accurate because as the acid concentration increases, Ka increases.
- Line C is the most accurate because as the acid concentration.
- Line C is the most accurate because as the acid concentration increase, Ka decreases.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the condensed structure of an isomer of this molecule:
CH3-CH=CH-CH3
PLEASE HELP NOW URGENT!
HELP NOW PLEASE URGENT
Chapter 16 Solutions
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
Ch. 16.2 - Practice Exercise 1 Consider the following...Ch. 16.2 - Prob. 16.1.2PECh. 16.2 - Prob. 16.2.1PECh. 16.2 - Practice Exercise 2 When lithium oxide (Li2O) is...Ch. 16.2 - Based on information in Figure 16.4, place the...Ch. 16.2 - Practice Exercise 2 For each reaction, use Figure...Ch. 16.3 - Practice Exercise 1 In a certain acidic solution...Ch. 16.3 - Practice Exercise 2 Indicate whether solutions...Ch. 16.3 - Prob. 16.5.1PECh. 16.3 - Prob. 16.5.2PE
Ch. 16.4 - Practice Exercise 1 A solution at 250C has [OH-] =...Ch. 16.4 - Practice Exercise 2 In a sample of lemon juice,...Ch. 16.4 - Practice Exercise 1 A solution at 25° C has pOH =...Ch. 16.4 - Prob. 16.7.2PECh. 16.5 - Practice Exercise 1 Order the following three...Ch. 16.5 - Prob. 16.8.2PECh. 16.5 - Practice Exercise 1 Order the following three...Ch. 16.5 - Prob. 16.9.2PECh. 16.6 - Prob. 16.10.1PECh. 16.6 - Practice Exercise 2 Niacin, one of the B vitamins,...Ch. 16.6 - Prob. 16.11.1PECh. 16.6 - Practice Exercise 2 A 0.020 M solution of niacin...Ch. 16.6 - Practice Exercise 1 What is the pH of a 0.40 M...Ch. 16.6 - Practice Exercise 2 The Ka for niacin (Sample...Ch. 16.6 - Prob. 16.13.1PECh. 16.6 - Prob. 16.13.2PECh. 16.6 - Practice Exercise 1 What is the pH of a 0.28 M...Ch. 16.6 - Practice Exercise 2 Calculate the pH of a 0.020 M...Ch. 16.7 - Prob. 16.15.1PECh. 16.7 - Practice Exercise 2 Which of the following...Ch. 16.7 - Prob. 16.16.1PECh. 16.7 - Practice Exercise 2 What is the morality of an...Ch. 16.8 - Practice Exercise 1 By using information from...Ch. 16.8 - Practice Exercise 2 Based on information in...Ch. 16.9 - Prob. 16.18.1PECh. 16.9 - Prob. 16.18.2PECh. 16.9 - Practice Exercise 1 How many of the following...Ch. 16.9 - Practice Exercise 2 Predict whether the...Ch. 16.10 - Prob. 16.20.1PECh. 16.10 - In each pair, choose the compound that gives the...Ch. 16 - Prob. 1DECh. 16 - a. Identify the Br ted-Lowry acid and base in the...Ch. 16 - Prob. 2ECh. 16 - Prob. 3ECh. 16 - Prob. 4ECh. 16 - 16.5 The following diagrams represent aqueous...Ch. 16 - Prob. 6ECh. 16 - Which of these statements about how the percent...Ch. 16 - 16.8 Each of the three molecules shown here...Ch. 16 - Prob. 9ECh. 16 - Which of the following diagrams best represent an...Ch. 16 - Prob. 11ECh. 16 - Prob. 12ECh. 16 - Prob. 13ECh. 16 - 16.14 Which of the following statements is...Ch. 16 - Prob. 15ECh. 16 - Prob. 16ECh. 16 - Identify the Bronsted-Lowry acid and the...Ch. 16 - Prob. 18ECh. 16 - Prob. 19ECh. 16 - Prob. 20ECh. 16 - Prob. 21ECh. 16 - Prob. 22ECh. 16 - Prob. 23ECh. 16 - Prob. 24ECh. 16 - Prob. 25ECh. 16 - Prob. 26ECh. 16 - Prob. 27ECh. 16 - Prob. 28ECh. 16 - 16.29 Calcualte [H +] for each of the following...Ch. 16 - Prob. 30ECh. 16 - 16.31 At the freezing point of water (0 o C), K10...Ch. 16 - Prob. 32ECh. 16 - Prob. 33ECh. 16 - Prob. 34ECh. 16 - 16.35 Complete the following table by calculating...Ch. 16 - Prob. 36ECh. 16 - Prob. 37ECh. 16 - 16.38 Carbon dioxide in the atmosphere dissolves...Ch. 16 - Prob. 39ECh. 16 - Prob. 40ECh. 16 - Prob. 41ECh. 16 - Prob. 42ECh. 16 - Prob. 43ECh. 16 - Prob. 44ECh. 16 - Prob. 45ECh. 16 - Prob. 46ECh. 16 - Prob. 47ECh. 16 - Prob. 48ECh. 16 - Prob. 49ECh. 16 - write the chemical equation and the Ka expression...Ch. 16 - Prob. 51ECh. 16 - Prob. 52ECh. 16 - Prob. 53ECh. 16 - Prob. 54ECh. 16 - Prob. 55ECh. 16 - Prob. 56ECh. 16 - Prob. 57ECh. 16 - Prob. 58ECh. 16 - Calculate the pH of each of the following solution...Ch. 16 - Prob. 60ECh. 16 - Prob. 61ECh. 16 - Prob. 62ECh. 16 - Calculate the percent ionization of hydrazoic acid...Ch. 16 - 16.64 Calculate the percent ionization of...Ch. 16 - Prob. 65ECh. 16 - Prob. 66ECh. 16 - Prob. 67ECh. 16 - 16.68 The hypochlorite ion, CIO- , acts as a weak...Ch. 16 - Prob. 69ECh. 16 - Prob. 70ECh. 16 - Calculate the molar concentration of OH- in a...Ch. 16 - 16.72 Calculate the molar concentration of OH- in...Ch. 16 - Prob. 73ECh. 16 - Prob. 74ECh. 16 - Prob. 75ECh. 16 - Prob. 76ECh. 16 - a. Given that Ka for acetic acid is 1.8 10-5 and...Ch. 16 - 16.78
a. Given that Kb for ammonia is 1.8 X 10 -5...Ch. 16 - Prob. 79ECh. 16 - Prob. 80ECh. 16 - Prob. 81ECh. 16 - Pyridinium bromide (C5H5NHBr) is a strong...Ch. 16 - Prob. 83ECh. 16 - Prob. 84ECh. 16 - Prob. 85ECh. 16 - 16.86 An unknown salt is either KBr, NH4 C1, KCN,...Ch. 16 - Prob. 87ECh. 16 - Prob. 88ECh. 16 - 16.89 Based on their compositions and structures...Ch. 16 - Prob. 90ECh. 16 - 16.91 Indicate whether each of the following...Ch. 16 - Prob. 92ECh. 16 - Prob. 93ECh. 16 - Prob. 94ECh. 16 - Prob. 95ECh. 16 - Prob. 96ECh. 16 - Prob. 97ECh. 16 - Prob. 98ECh. 16 - Prob. 99AECh. 16 - Prob. 100AECh. 16 - Prob. 101AECh. 16 - Prob. 102AECh. 16 - Prob. 103AECh. 16 - Prob. 104AECh. 16 - Benzoic acid (C6H5COOH) and aniline (C6H5NH2) are...Ch. 16 - Prob. 106AECh. 16 - Prob. 107AECh. 16 - Prob. 108AECh. 16 - Butyric acid is responsible for the foul smell of...Ch. 16 - Prob. 110AECh. 16 - Prob. 111AECh. 16 - Prob. 112AECh. 16 - 1S.113 Many moderately large organic molecules...Ch. 16 - Prob. 114AECh. 16 - Prob. 115AECh. 16 - Prob. 116IECh. 16 - Prob. 117IECh. 16 - Prob. 118IECh. 16 - Prob. 119IECh. 16 - 16.120 At 50 oC, the ion-product constant for H2...Ch. 16 - Prob. 121IECh. 16 - Prob. 122IECh. 16 - Prob. 123IECh. 16 - Prob. 124IECh. 16 - Prob. 125IECh. 16 - Prob. 126IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How do I solve this Alkyne synthesis homework problem for my Organic Chemistry II class? I have to provide both the intermediate products and the reagents used.arrow_forwardSubstance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point enthalpy of fusion 90. °C 8.00 kJ/mol boiling point 130. °C enthalpy of vaporization 44.00 kJ/mol density 2.80 g/cm³ (solid) 36. J.K mol (solid) 2.50 g/mL (liquid) heat capacity 32. J.Kmol (liquid) 48. J.Kmol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Ex Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 15.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. o0o 150- 140 130- 120- 110- 100- G Ar ?arrow_forwardMechanism. Provide the mechanism for the reaction below. You must include all arrows, intermediates, and formal charges. If drawing a Sigma complex, draw all major resonance forms. The ChemDraw template of this document is available on Carmen. Br FeBr3 Brarrow_forward
- Check the box under each compound that exists as a pair of mirror-image twins. If none of them do, check the none of the above box under the table. CH3 OH CH3 CH2 -CH-CH3 CH3 OH OH CH-CH2-CH- -CH3 CH3 CH3 OH OH CH3 C -CH2- C. -CH3 CH3- -CH2- -CH-CH2-OH OH CH3 none of the above كarrow_forwardWrite the systematic name of each organic molecule: structure Η OH OH OH OH H namearrow_forwardDraw the skeletal ("line") structure of a secondary alcohol with 5 carbon atoms, 1 oxygen atom, at least one ring, and no double or triple bonds. Click and drag to start drawing a structure. : ☐ ☑ ⑤arrow_forward
- Name these organic compounds: structure name CH₁₂ CH3 - C CH - CH2 || CH3- - CH₂ CH₂ | - - CH3 CH3 2-methyl-2-butene ☐ 3-methyl-1-butyne - CH3 CH. - C=CHarrow_forwardHow many different molecules are drawn below?arrow_forwardWith the reference to a anion A, Label compounds B-F as an isomer or resonance strcuture of A. FOr each isomer indicate what bonds differs from A. Provide steps and undertanding on how you come up with work.arrow_forward
- Provide steps and also tips to undertand how to do on my own. Add the correct number of hydrogen atoms for each carbon atom and lone pairs to each oxygen atom.arrow_forwardA mixture of oxygen and ethyne is burnt for welding tell why mixture of ethyne and air is not usedarrow_forwardQ2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY