
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 67P
Synthesize each compound from benzene and any other organic or inorganic reagents.
a. 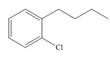 c.
c. 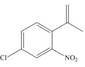 e.
e. 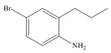 g.
g. 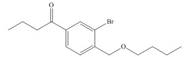
b. 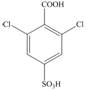 d.
d. 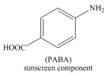 f.
f. 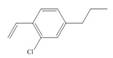 h.
h. 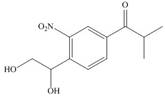
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 16 Solutions
Organic Chemistry (6th Edition)
Ch. 16.1 - Prob. 1PCh. 16.2 - Prob. 2PCh. 16.3 - Prob. 3PCh. 16.4 - Prob. 4PCh. 16.5 - Prob. 5PCh. 16.5 - Prob. 6PCh. 16.5 - Prob. 9PCh. 16.5 - Problem 18.9 Draw the product of each reaction
a....Ch. 16.5 - Prob. 11PCh. 16.5 - Prob. 12P
Ch. 16.5 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Problem 18.14 Draw all resonance structures for...Ch. 16.6 - Problem 18.15 Classify each substituent as...Ch. 16.7 - Prob. 17PCh. 16.9 - Prob. 22PCh. 16.10 - Problem 18.20 Draw the products of each...Ch. 16.10 - Prob. 24PCh. 16.11 - Problem 18.22 Draw the products formed when each...Ch. 16.12 - Problem 18.23 Devise a synthesis of each compound...Ch. 16.13 - Problem 18.24 Draw the products of each...Ch. 16.13 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 16.13 - Problem 18.26 Draw the products of each...Ch. 16.13 - Prob. 30PCh. 16 - Prob. 37PCh. 16 - 18.35 What is the major product formed by an...Ch. 16 - 18.36 Draw the products formed when phenol is...Ch. 16 - Problem 18.37 Draw the products formed when each...Ch. 16 - 18.38 Draw the products of each reaction.
a. d....Ch. 16 - 18.39 What products are formed when benzene is...Ch. 16 - Prob. 49PCh. 16 - 18.47 For each of the following substituted...Ch. 16 - 18.48 Consider the tetracyclic aromatic compound...Ch. 16 - 18.49 For each N-substituted benzene, predict...Ch. 16 - Prob. 54PCh. 16 - 18.51 Using resonance structures, explain why a...Ch. 16 - Prob. 56PCh. 16 - 18.53 Rank the aryl halides in each group in order...Ch. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - Prob. 66PCh. 16 - 18.63 Synthesize each compound from benzene and...Ch. 16 - Problem 18.64 Synthesize each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY