
a.
Interpretation:
Name the given compound.
Concept introduction:
The nomenclature of
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:
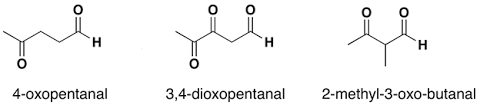
Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:
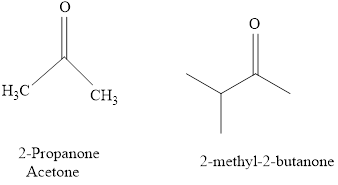
b.
Interpretation:
Name the given compound.
Concept introduction:
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:

Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

c.
Interpretation:
Name the given compound.
Concept introduction:
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:

Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

d.
Interpretation:
Name the given compound.
Concept introduction:
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of an aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
The common name of the aldehyde is substituted for "oic acid" at the end of parent hydrocarbon chain.
For example:

Naming Ketones:
Carbonyl carbon atom attached to the two oxygen atoms.
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Student's Study Guide and Solutions Manual for Organic Chemistry
- DATA: Standard Concentration (caffeine) mg/L Absorbance Reading 10 0.322 20 0.697 40 1.535 60 2.520 80 3.100arrow_forwardIn what position will p-Toluidine be nitrated and what will the compound be called.arrow_forwardIn what position will 4-methylbenzonitrile be nitrated and what will the compound be called.arrow_forward
- In what position will benzenesulfonic acid be nitrated?arrow_forwardIf compound A reacts with an excess of methyl iodide and then heated with aqueous Ag₂O, indicate only the major products obtained. Draw their formulas. A Harrow_forwardExplanation Check 1:01AM Done 110 Functional Groups Identifying and drawing hemiacetals and acetals In the drawing area below, create a hemiacetal with 1 ethoxy group, 1 propoxy group, and a total of 9 carbon atoms. Click and drag to start drawing a structure. ✓ $ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Sarrow_forward
- Write the systematic name of each organic molecule: CI structure CI CI Explanation CI ठ CI Check B ☐ 188 F1 80 name F2 F3 F4 F5 F6 60 F7 2arrow_forwardWrite the systematic name of each organic molecule: structure i HO OH Explanation Check name ☐ ☐arrow_forwardX 5 Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. CI Br Br Br 0 None of these molecules have a total of five ẞ hydrogens. Explanation Check esc F1 F2 tab caps lock fn Q @2 A W # 3 OH O OH HO © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility IK F7 F7 F8 TA F9 F10 & 6 28 * ( > 7 8 9 0 80 F3 O F4 KKO F5 F6 S 64 $ D % 25 R T Y U பட F G H O J K L Z X C V B N M H control option command P H F11 F12 + || { [ command optionarrow_forward
- An open vessel containing water stands in a laboratory measuring 5.0 m x 5.0 m x 3.0 m at 25 °C ; the vapor pressure (vp) of water at this temperature is 3.2 kPa. When the system has come to equilibrium, what mass of water will be found in the air if there is no ventilation? Repeat the calculation for open vessels containing benzene (vp = 13.1 kPa) and mercury (vp = 0.23 Pa)arrow_forwardEvery chemist knows to ‘add acid to water with constant stirring’ when diluting a concentrated acid in order to keep the solution from spewing boiling acid all over the place. Explain how this one fact is enough to prove that strong acids and water do not form ideal solutions.arrow_forwardThe predominant components of our atmosphere are N₂, O₂, and Ar in the following mole fractions: χN2 = 0.780, χO2 = 0.21, χAr = 0.01. Assuming that these molecules act as ideal gases, calculate ΔGmix, ΔSmix, and ΔHmix when the total pressure is 1 bar and the temperature is 300 K.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





