
Concept explainers
Compounds Y and Z are isomers with the molecular formula
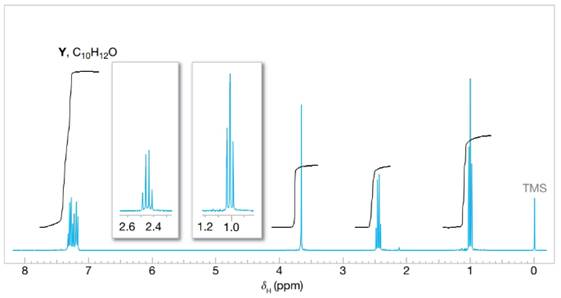
FIGURE 16.4 The
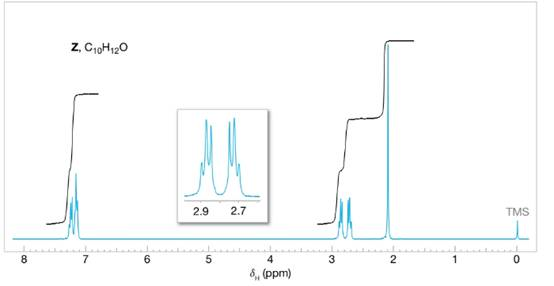
FIGURE 16.5 the
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY-WILEYPLUS ACCESS PKG.
Additional Science Textbook Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Introductory Chemistry (6th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Chemistry (7th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Indicate the functions that salt bridges have in batteries.arrow_forwardIn the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.arrow_forwardWrite the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?arrow_forward
- State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forward
- Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forwardhelp me solve this hwarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

