
Concept explainers
(a)
Interpretation:
The length of the face diagonal in terms of
Concept introduction:
The crystal can exist in various shapes but the most common one is cubic. The FCC unit cell has lattice points at eight corners of the unit cell along with the face centers of unit cell. The total number of atoms in FCC unit cell is four.
Answer to Problem 1ASA
The length of the face diagonal in terms of
Explanation of Solution
The face diagonal BC, represented by a, is shown below.
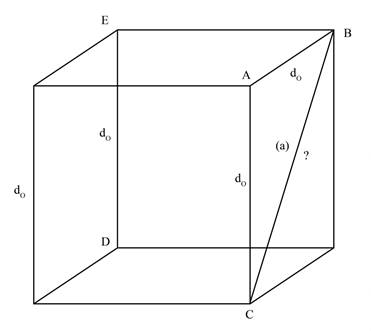
Figure 1
In the Figure 1, the face diagonal, represented by a, is the hypotenuse of right angle triangle ABC. The given unit cell is cube. Therefore, the length of AB will be equal to length of AC.
Therefore,
The length of face diagonal is calculated by using Pythagoras theorem as shown below.
Substitute the value of AB and AC in equation (1)
Therefore, the length of the face diagonal in terms of
The length of the face diagonal in terms of
(b)
Interpretation:
The length of the cube diagonal in terms of
Concept introduction:
The crystal can exist in various shapes but the most common one is cubic. The FCC unit cell has lattice points at eight corners of the unit cell along with the face centers of unit cell. The total number of atoms in FCC unit cell is four.
Answer to Problem 1ASA
The length of the cube diagonal in terms of
Explanation of Solution
A line that runs from one corner to other corner of the cube via centre of the cube is termed as cube diagonal. The cube diagonal is represented by CE as shown below.
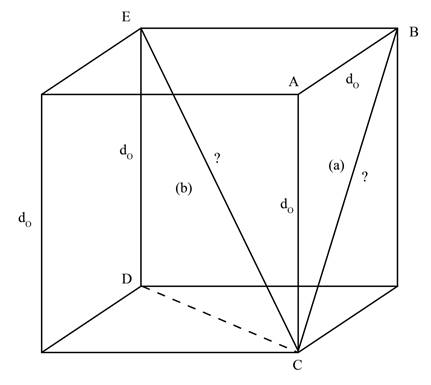
Figure 2
In the Figure 2, the cube diagonal, represented by b, is the hypotenuse of right angle triangle CDE. The edge length DE is given as
Therefore,
The length of face diagonal is calculated by using Pythagoras theorem as shown below.
Substitute the value of edge length DE and face diagonal CD in equation (2).
Therefore, the length of the cube diagonal in terms of
The length of the cube diagonal in terms of
Want to see more full solutions like this?
Chapter 16 Solutions
EBK CHEMICAL PRINCIPLES IN THE LABORATO
- Is (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forwardThree pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forward
- Draw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





