
(a)
Interpretation:
The given reaction is
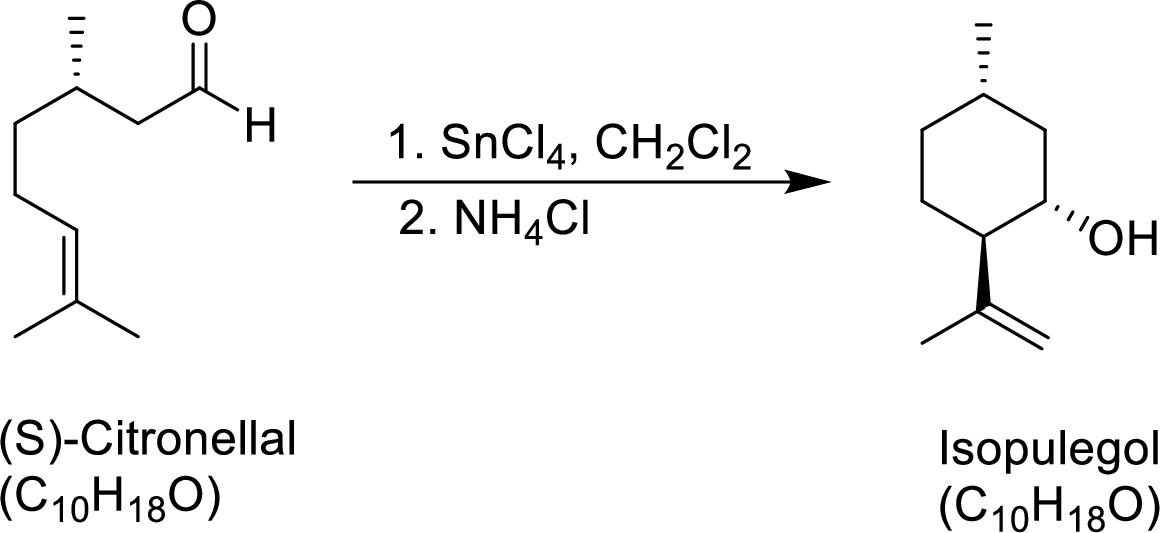
Both compounds has to be showed as terpenes.
Concept Introduction:
Terpene:
The compounds which contains two or more isoprene units in their structure are said to be terpenes.
Isoprene (2-methyl-1,3-butadiene) unit is a molecule with five carbon atoms with double bonds.
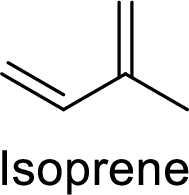
(b)
Interpretation:
A mechanism for the conversion of (S)-Citronellal to Isopulegol has to be proposed.
(c)
Interpretation:
In Isopulegol, the number of stereocenters present has to be given and the number of stereoisomers possible for a molecule with this number of stereocenters has to be given.
Concept Introduction:
A carbon which is bonded to four different groups is said to be chiral carbon. A chiral carbon in a molecule is said to be chiral center. All chiral centers are stereocenters.
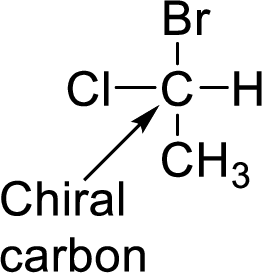
(d)
Interpretation:
Isopulegol is formed as a single stereoisomer. The fact that only single stereoisomer is formed has to be accounted.
Trending nowThis is a popular solution!

Chapter 16 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

