
Concept explainers
(a)
Interpretation:
Given sugar below is a D- or L- sugar has to be determined.
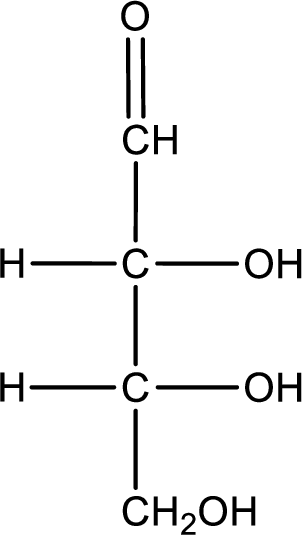
Concept Introduction:
Chiral compounds are optically active and they rotate the plane polarized light. Some compounds rotate the plane polarized light clockwise and some rotate it anticlockwise. Compounds that rotate clockwise are known as dextrorotatory and those which rotate anticlockwise are known as levorotatory. Dextrorotatory compounds are indicated with a “(+)” sign while levorotatory compounds are indicated with a “(-)” sign. In case of sugar, the chiral carbon that contains hydroxyl group farthest from the most oxidized end of the molecule is considered. If the hydroxyl group is present on the right side, then the isomer is said to have D-configuration and if it is present on the left side, then the isomer is said to be in L-configuration.
(b)
Interpretation:
Given sugar below is a D- or L- sugar has to be determined.
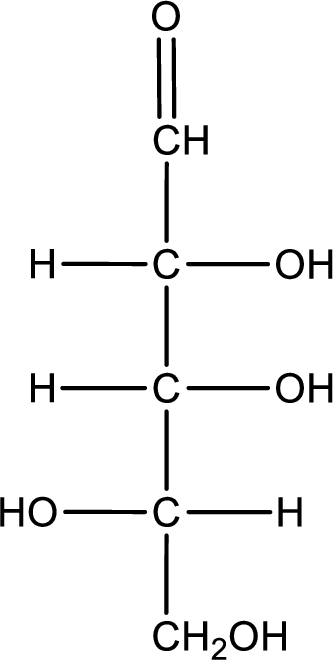
Concept Introduction:
Refer part (a).
(c)
Interpretation:
Given sugar below is a D- or L- sugar has to be determined.
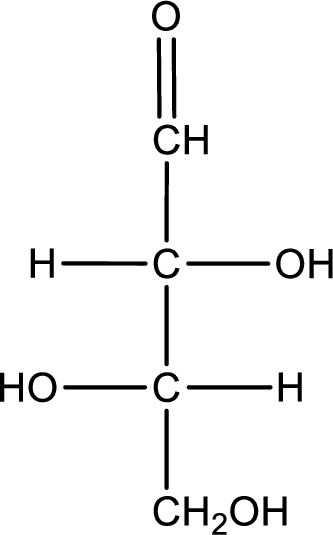
Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
GENERAL,ORGANIC+BIOCHEM (LOOSELEAF)
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





