
To sketch:
A titration curve for the titration of
a.
b.
c.
d.
Answer to Problem 16.46QA
Solution:
a)
b)
c)
d)
Explanation of Solution
1) Concept:
Oxalic acid is a weak diprotic acid, i.e., there are two protons that can dissociate from one molecule of oxalic acid in aqueous solution. The dissociation of protons occurs stepwise.
The first equivalence point occurs where the first dissociated proton is completely neutralized when titrated with a strong base
The second equivalence point occurs where both dissociated protons fully neutralized, and the equation is
Equation for complete titration is
At equivalence point, the equal moles of weak acid and strong base react and undergo neutralization. The second
2) Formula:
i)
ii)
iii)
iv)
v)
3) Given:
i) Molarity of oxalic acid,
ii) Volume of oxalic acid,
iii) Molarity of
iv)
v)
vi) pKa1 = 1.23 (Appendix 5 Table A5.1)
vii) pKa2 = 4.19 (Appendix 5 Table A5.1)
4) Calculation:
Converting volume in
Calculating moles of oxalic acid
These are the initial moles of oxalic acid present before any
Now, calculating the
a)
Converting volume in ml to L,
Calculating the moles of
Set up the RICE table that is used to calculate the moles of acid reacted and moles of conjugate base formed.
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
b)
Converting volume in ml to L,
Calculating the moles of
Setting up the RICE table to calculate the moles of acid reacted and moles of conjugate base formed,
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Moles of the weak acid and conjugate base are equal.Thisis the half equivalence point to the first equivalence point, and the pH at half equivalence point is
c)
Converting volume in ml to L,
Calculating the moles of
Setting up the RICE that is used to calculate the moles of acid reacted and moles of conjugate base formed,
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
Calculating the concentration of
d)
Converting volume in ml to L,
Calculating the moles of
Set up the RICE table to calculate the moles of acid reacted and moles of conjugate base formed.
| Reaction | + | + | |||||
| Initial | 0 | ||||||
| Change | |||||||
| Final | 0 | ||||||
This is the first equivalence point since no moles of weak acid or strong base are left.
There is not much difference in the
At the equivalence point, the major species present is
Total volume of solution
Molarity of
| Initial (M) | |||
| Change (M) | |||
| Equilibrium (M) | |||
Writing the
A titration curve for the titration of
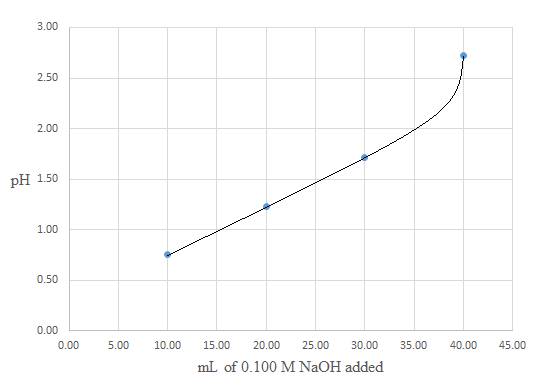
Conclusion:
Using the RICE table, we can determinethe
Want to see more full solutions like this?
Chapter 16 Solutions
CHEM:ATOM FOC 2E CL (TEXT)
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





