
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
8th Edition
ISBN: 9780135204634
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.45CP
Look at the electron-dot structures of the following molecules and ions:
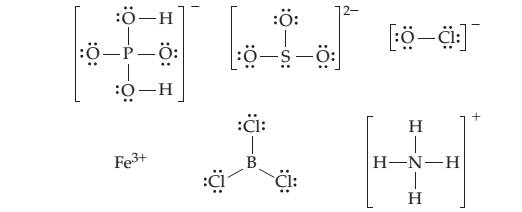
(a) Which of these molecules and ions can behave as a Bronsted—Lowry acid? Which can behave as a Bronsted— Lowry base?
(b) Which can behave as a Lewis acid? Which can behave as a Lewis base?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
Don't used hand raiting
Can you please explain why the correct answer for this question is letter B? I chose letter A because I thought that a kinetic product was a 1,2-addition. Please give a detailed explanation.
Chapter 16 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
Ch. 16 - Write a balanced equation for the dissociation of...Ch. 16 - Write the reaction between the carbonate ion...Ch. 16 - Conceptual PRACTICE 15.3 For the following...Ch. 16 - Conceptual APPLY 15.4 For the following reactions...Ch. 16 - If you mix equal concentrations of reactants and...Ch. 16 - Conceptual APPLY 15.6 The following pictures...Ch. 16 - Which pair has the stronger acid listed first? H2S...Ch. 16 - Which acid is stronger, H3PO4orH3AsO4?Ch. 16 - PRACTICE 15.9 The concentration of H3O+ ions in...Ch. 16 - Calculate the pH of a sample of seawater that has...
Ch. 16 - During mining operations, the mineral pyrite...Ch. 16 - Calculate the concentrations of H3O+ and OH- in a...Ch. 16 - Calculate the pH of the following solutions: (a)...Ch. 16 - Calculate the pH of a solution prepared by...Ch. 16 - The following pictures represent aqueous solutions...Ch. 16 - Acetic acid, CH3CO2H, is the solute that gives...Ch. 16 - Wha concentration of formic acid will result in a...Ch. 16 - Calculate the pH and the concentration of all...Ch. 16 - Carbonated drinks are prepared by dissolving CO2...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Lactated Ringers solution is given intravenously...Ch. 16 - Prob. 16.25PCh. 16 - The following pictures represent aqueous solutions...Ch. 16 - Predict whether a solution of 0.20 M NaNO2 is...Ch. 16 - Calculate the pH and percent dissociation of...Ch. 16 - Prob. 16.29PCh. 16 - For the following Lewis acid— base reaction, draw...Ch. 16 - What are the chemical formulas and names of the...Ch. 16 - What were the average pH ranges for rainfall in...Ch. 16 - Prob. 16.33PCh. 16 - (a) Natural or “unpolluted” rain has a pH of 5.6....Ch. 16 - Prob. 16.35PCh. 16 - Prob. 16.36PCh. 16 - Because sulfur and nitrogen oxides are the main...Ch. 16 - Prob. 16.38CPCh. 16 - The following pictures represent aqueous solutions...Ch. 16 - Locate sulfur, selenium, chlorine, and bromine in...Ch. 16 - Prob. 16.41CPCh. 16 - Prob. 16.42CPCh. 16 - The followign pictures represent solutions of...Ch. 16 - Prob. 16.44CPCh. 16 - Look at the electron-dot structures of the...Ch. 16 - Boric acid (H3BO3) is a weak monoprotic acid that...Ch. 16 - Prob. 16.47CPCh. 16 - Prob. 16.48SPCh. 16 - Which of the following can behave both as a...Ch. 16 - Give the formula for the conjugate base of each of...Ch. 16 - Give the formula for the conjugate acid of each of...Ch. 16 - For each of the following reactions, identify the...Ch. 16 - For each of the following reactions, identify the...Ch. 16 - Aqueous solutions of hydrogen sulfide contain...Ch. 16 - Prob. 16.55SPCh. 16 - Choose from the conjugate acid-base pairs...Ch. 16 - Prob. 16.57SPCh. 16 - Prob. 16.58SPCh. 16 - Prob. 16.59SPCh. 16 - Arrange each group of compounds in order of...Ch. 16 - Arrange each group of compounds in order of...Ch. 16 - Prob. 16.62SPCh. 16 - Identify the weakest acid in each of the following...Ch. 16 - Prob. 16.64SPCh. 16 - Identify the stronger base in each of the...Ch. 16 - Prob. 16.66SPCh. 16 - Prob. 16.67SPCh. 16 - The concentration of OH- in a sample of seawater...Ch. 16 - The concentration of OH- in human blood is...Ch. 16 - For each of the following solutions, calculate...Ch. 16 - For each of the following solutions, calculate...Ch. 16 - Water superheated under pressure to 200oC and 750...Ch. 16 - Water at 500oC and 250 atm is a supercritical...Ch. 16 - Calculate the pH to the correct number of...Ch. 16 - Calculate the pH to the correct number of...Ch. 16 - Calculate the H3O+ concentration to the correct...Ch. 16 - Calculate the H3O+ concentration to the correct...Ch. 16 - Prob. 16.78SPCh. 16 - Which of the indicators given in Figure 16.5,...Ch. 16 - Which of the following species behave a strong...Ch. 16 - Which of the following species behave as strong...Ch. 16 - Calculate the pH of the following solutions:...Ch. 16 - Calculate the pH of the following solutions: 0.48...Ch. 16 - Prob. 16.84SPCh. 16 - Calculate the pH of solutions prepared by: RAN (a)...Ch. 16 - How many grams of CaO should be dissolved in...Ch. 16 - Prob. 16.87SPCh. 16 - Look up the value of Ka in Appendix C for...Ch. 16 - Look up the value of Ka in Appendix C for...Ch. 16 - The pH of 0.040 M hypobromous acid (HOBr) is 5.05....Ch. 16 - Lactic acid (C3H6O3) , which occurs in sour milk...Ch. 16 - The pH of 0.050 M gallic acid, an acid found in...Ch. 16 - The pH of 0.040 M pyruvic acid, an acid found in...Ch. 16 - A vitamin C tablet containing 250 mg of ascorbic...Ch. 16 - Acetic acid (CH3COOH;Ka=1.810-5) has a...Ch. 16 - Acrylic acid (HC3H3O2) is used in the manufacture...Ch. 16 - Hippuric acid (HC9H8NO3) , found in horse urine,...Ch. 16 - Calculat the pH and the percent dissociation in...Ch. 16 - A typical aspirin tablet contains 324 mg of...Ch. 16 - Prob. 16.100SPCh. 16 - Calculate the percent dissociation of...Ch. 16 - Write balanced net ionic equations and the...Ch. 16 - Write balanced net ionic equations and the...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Prob. 16.105SPCh. 16 - Prob. 16.106SPCh. 16 - Tartaric acid (C4H6O6) is a diprotic acid that...Ch. 16 - Like sulfuric acid, selenic acid (H2SeO4) is a...Ch. 16 - Calculate the concentrations of H3O+ and SO42- in...Ch. 16 - Prob. 16.110SPCh. 16 - Prob. 16.111SPCh. 16 - Write a balanced net ionic equation and the...Ch. 16 - Write a balanced net ionic equation and the...Ch. 16 - Styrchine (C21H22N2O2) , a deadly poison used for...Ch. 16 - What is the pH of 0.5 M ammonia (NH3)?(Kb=1.8105)Ch. 16 - Morphine (C17H19NO3), a narcotic used in...Ch. 16 - A 1.00103M solution of quinine, a drug used in...Ch. 16 - Oxycodone (C18H21NO4), a narcotic analgesic, is a...Ch. 16 - Morpholine (C4H9NO) is a weak organic base with...Ch. 16 - Using values of Kb in Appendix C, calculate values...Ch. 16 - Using values of Ka in Appendix C, calculate values...Ch. 16 - Prob. 16.122SPCh. 16 - Sodium benzoate (C6H5CO2Na) is used as a food...Ch. 16 - Write a balanced net ionic equation for the...Ch. 16 - Write a balanced net ioflk equation for the...Ch. 16 - Classify each of the following ions according to...Ch. 16 - Classify each of the following salt solutions as...Ch. 16 - Calculate the concentrations of all species...Ch. 16 - Prob. 16.129SPCh. 16 - Calculate Ka for the cation Kb for the anion in an...Ch. 16 - Classify each of the following salt solutions as...Ch. 16 - Prob. 16.132SPCh. 16 - Classify each of the following salt solutions as...Ch. 16 - Calculate the pH and the concentrations of all...Ch. 16 - Calculate the pH and the percent dissociation of...Ch. 16 - Prob. 16.136SPCh. 16 - Prob. 16.137SPCh. 16 - Prob. 16.138SPCh. 16 - For each of the following reactions, identify the...Ch. 16 - Prob. 16.140SPCh. 16 - For each of the Lewis acid—base reactions in...Ch. 16 - Prob. 16.142SPCh. 16 - Prob. 16.143SPCh. 16 - Prob. 16.144MPCh. 16 - Prob. 16.145MPCh. 16 - Prob. 16.146MPCh. 16 - Prob. 16.147MPCh. 16 - Normal rain has a pH of 5.6 due to dissolved...Ch. 16 - Sulfur dioxide is quite soluble in water:...Ch. 16 - Prob. 16.150MPCh. 16 - Acid and base behavior can be observed in solvents...Ch. 16 - Prob. 16.152MPCh. 16 - In the case of very weak acids, [H3O+] from the...Ch. 16 - Prob. 16.154MPCh. 16 - Prob. 16.155MPCh. 16 - Neutralization reactions involving either a strong...Ch. 16 - Prob. 16.157MPCh. 16 - Prob. 16.158MPCh. 16 - A 200.0 mL sample of 0.350 M acetic acid (CH3CO2H)...Ch. 16 - Prob. 16.160MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please explain why the answer is structures 2 and 3? Please include a detailed explanation and show how the synthesis can be done with those two structures.arrow_forwardCan you please explain why the correct answer to this question is option 2? I am having trouble understanding how and why. Please provide a detailed explanation and a drawing of how the diene and dienophile would create the product in the question.arrow_forwardCan you please explain why the correct answer is molecules 2 and 4? Base your explanation off of the rules for aromaticity and well as the principles of the Huckel rule of aromaticity. Please give a detailed explanation of what Hucekl's rule is.arrow_forward
- Can you please explain why the answer is B and not A? I chose A because I thought the thermodynamic product was a 1,4-addition. Please give a detailed explanation to this problem and include a drawing of how the reaction works.arrow_forwardLabel the diagram according to the components and processes of an alkaline batteryarrow_forwardCan you please explain why the answer to the question is option 4? Please include the aromaticity rules as well as Huckel's rule. Please label molecules 1, 2, 3, and 5 with their respective labels of aromatic or nonaromatic and why.arrow_forward
- Don't used hand raitingarrow_forwardCan you please explain why the correct answer is molecules 2 and 4? Please provide a detailed explanation as well as the two molecules drawn showing what and where it is conjugated.arrow_forwardCan you please explain why the correct answer is (2E, 4Z, 6Z)-2,4,6-Nonatriene? Please include a detailed explanation and a drawing of the structure, with the corresponding parts of the answer labeled. I'm confused why 6 is Z and why it is Nonatriene.arrow_forward
- ? /1600 O Macmillan Learning Using the data in the table, determine the rate constant of the Trial [A] (M) [B] (M) Rate (M/s) reaction and select the appropriate units. 1 0.240 0.350 0.0187 2 0.240 0.700 0.0187 A+2B C+D 3 0.480 0.350 0.0748 k = Unitsarrow_forwardCan you please explain why structure 3 is the correct answer? I am having trouble understanding why it is aromatic. Can you also label molecules 1, 2, 4, and 5 with the correct nonaromatic or antiaromatic?arrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY