
Concept explainers
Interpretation:
The pH of the titration of
Explanation of Solution
Given,
Titration of
Addition of
Given system is titration of
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | Excess |
The total volume of the given solution is 1 ml + 0.25 ml = 1.25 ml (or) 0.0625 L
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | Excess |
The total volume of the given solution is 1 ml + 0.50 ml = 1.50 ml (or) 0.0015 L
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | 0 | Excess |
The total volume of the given solution is 1 ml + 1.2 ml = 2.20 ml (or) 0.0022 L
The resulting solution is neutral. Therefore,
Hence,
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | Excess |
The total volume of the given solution is 1 ml + 1.5 ml = 2.5 ml (or) 0.0025 L
The concentration of
The titration curve is given below,
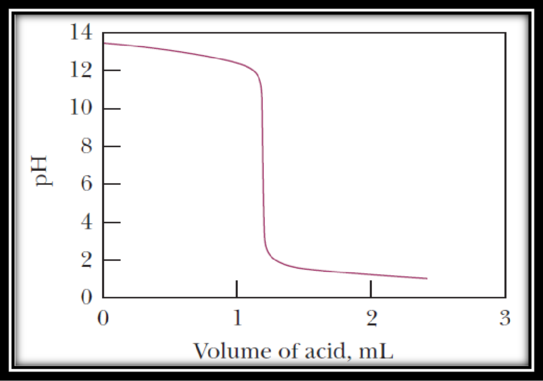
Figure 1
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry: Principles and Practice
- ogin - PaymentN MapQuest 3 Overview - SAP NetW... Draw the major product of this reaction. Ignore inorganic byproducts. CI 1. NaBH4 2. H₂O C Clever | Portal Job Op Problem Atoms, Bonds and Rings Draw or tap a new bond toarrow_forward2. Draw the remaining two resonance structures for the carbocation intermediate in the meta nitration of methyl benzoate AND explain why the meta orientation is preferred. Hint: how is the placement of the cation favorable after addition of nitronium relative to the electron withdrawing group? (2 pts) H NO2 CO₂Mearrow_forwardLabel all absorptions over 1500 cm-1. Please be specific and mark IR if needed for explanation. Compound OH sp^3 C-H C=O C-O Triglyceridearrow_forward
- Identify the intermediate that is INITIALLY formed in a saponification reaction (hydrolysis of an ester). III -OH H₂O HO OH HO O || A B C III D IV IVarrow_forwardHelp me answer this practice sheet I found for an answer guidearrow_forwardshow the retrosynthesis of this molecule step by step starting with 1,3-dimethoxy benzene H3CO OH OH OCH 3arrow_forward
- Consider the reaction of a propanoate ester with hydroxide ion shown below. A series of four alcohol leaving groups were tested to determine which would be the best leaving group. Based on the pKa values of the alcohols, predict which alcohol would produce the fastest hydrolysis reaction. HO FOR A Alcohol I, pKa =16.0 B Alcohol II, pKa =10.0 C Alcohol III, pKa = 7.2 + ROH D Alcohol IV, pKa = 6.6arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: NaOH, H₂O 00:4 Na O heat NaO Select to Add Arrows Select to Add Arrows :0: Na a NaOH, H2O :0: NaOH, H2O heat heat Na ONH Select to Add Arrowsarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H CH3NH3+ :0: :0: HO CH3NH2 HH iSelect to Add Arrows i Select to Add Arrows i HH CH3NH3+ CH3NH2 Select to Add Arrows i CH3NH3 CH3NH2 ايكدا HH Select to Add Arrowsarrow_forward
- The reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





