
Concept explainers
a Draw a pH titration curve that represents the titration of 25.0 mL of 0.15 M propionic acid. CH3CH2COOH, by the addition of 0.15 M KOH from a buret. Label the axes and put a scale on each axis. Show where the equivalence point and the buffer region are on the titration curve. You should do calculations for the 0%, 50%, 60%, and 100% titration points. b Is the solution neutral, acidic, or basic at the equivalence point? Why?
(a)
Interpretation:
For titration of 25.0 mL of 0.15 M propionic acid,
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 50%, 60% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.120QP
A pH titration curve showing the equivalence point and buffer region is given in Figure 1 as follows,
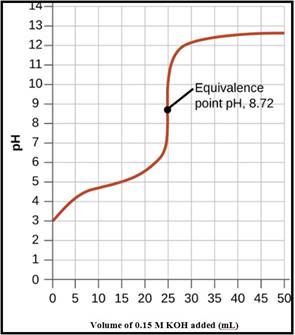
Figure 1
(a)
The pH at the 0% titration point is 2.85
The pH at the 50% titration point is 4.89
The pH at the 60% titration point is 5.06
The pH at the 100% titration point is 8.88
Explanation of Solution
To Draw: A pH titration curve showing the equivalence point and buffer region
The pH titration curve for the titration of 0.15 M propionic acid with 0.15 M
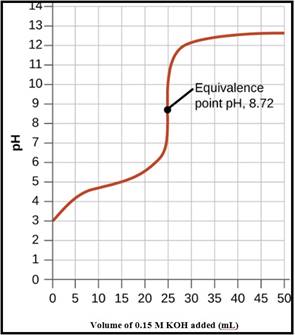
Figure 1
(a)
To Calculate: The pH of the titration points for the 0%, 50 %, 60% and 100%
Given data:
Titration of 0.15 M propionic acid with 0.15 M
pH at the 0% titration point:
Construct an equilibrium table with x as unknown concentration
Consider propionic acid as
|
|
|||
| Initial |
0.15
0.15-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Substitute equilibrium concentrations into the equilibrium-constant equation.
The
Assume x is negligible compared to 0.15 M
Therefore, the concentration of hydronium ion
In the end, pH is calculated as follows,
Therefore, the pH at the 0% titration point is 2.85
pH at the 50% titration point:
The pH is calculated as follows,
Therefore, the pH at the 50% titration point is 4.89
pH at the 60% titration point:
For convenience, express the concentrations as percents.
Substitute the concentrations into the equilibrium expression.
Therefore, the concentration of hydronium ion
In the end, pH is calculated as follows,
Therefore, the pH at the 60% titration point is 5.06
pH at the 100% titration point:
The salt that got produced has undergone a twofold dilution.
Therefore,
Construct an equilibrium table with x as unknown concentration
|
|
|||
| Initial |
0.0750
0.0750-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Now, calculate
Substitute into the equilibrium constant expression.
Here, x gives the concentration of hydroxide ion,
The pH is calculated as follows,
Therefore, the pH at the 100% titration point is 8.88
The pH at the 0% titration point was calculated as 2.85
The pH at the 50% titration point was calculated as 4.89
The pH at the 60% titration point was calculated as 5.06
The pH at the 100% titration point was calculated as 8.88
(b)
Interpretation:
For titration of 25.0 mL of 0.15 M propionic acid,
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 50%, 60% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.120QP
The solution at the equivalence point is basic
Explanation of Solution
To Explain: Whether the solution at the equivalence point is neutral, acidic or basic
As a result of titration Potassium propionate salt is produced.
Potassium propionate is the salt of a weak acid and a strong base.
Propionate ion reacts with water to produce hydroxide ions.
Therefore, the given solution is basic
The solution at the equivalence point was found as basic
Want to see more full solutions like this?
Chapter 16 Solutions
Bundle: General Chemistry, Loose-Leaf Version, 11th + LabSkills PreLabs v2 for Organic Chemistry (powered by OWLv2), 4 terms (24 months) Printed ... for Ebbing/Gammon's General Chemistry, 11th
- Show work. Don't give Ai generated solutionarrow_forwardExplain how the equation 4Fe(OH)2(s)+O2(g)→2Fe2O3(s)+4H2O(l) in the article illustrates the oxidation of the iron in the rectants.arrow_forwardIf you wanted to make something out of metal but didn't want it to rust, what are your options?arrow_forward
- Explain how the equation 4Fe(OH)2(s) + O2(g)→2Fe2O3(s) + 4H2O(l) in the article illustrates the oxidation of the iron ions in the reactantsarrow_forwardA Predict the major products of the following reaction. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. + Cl₂ 2 X Click and drag to start drawing a structure.arrow_forwardC app.aktiv.com Predict reagents needed to complete this E2 elimination reaction. Br Problem 17 of 40 H3O+ A heat NaH B heat 0 D E (CH)COK heat CH₂ONa (CH)COH heat Donearrow_forward
- Please correct answer and don't use hand ratingarrow_forwardDraw the structure of the product of this reaction. H CH2CH3 Br H-... H H3C KOH E2 elimination product • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If there are alternative structures, draw the most stable one. • If no reaction occurs, draw the organic starting material. O + 98 // n ?arrow_forward4. a) Give a suitable rationale for the following cyclization, stating the type of process involved (e.g. 9-endo-dig), clearly showing the mechanistic details at each step. H CO₂Me 1) NaOMe 2) H3O® CO₂Mearrow_forward
- 2. Platinum and other group 10 metals often act as solid phase hydrogenation catalysts for unsaturated hydrocarbons such as propylene, CH3CHCH2. In order for the reaction to be catalyzed the propylene molecules must first adsorb onto the surface. In order to completely cover the surface of a piece of platinum that has an area of 1.50 cm² with propylene, a total of 3.45 x 10¹7 molecules are needed. Determine the mass of the propylene molecules that have been absorbed onto the platinum surface.arrow_forwardChem 141, Dr. Haefner 2. (a) Many main group oxides form acidic solutions when added to water. For example solid tetraphosphorous decaoxide reacts with water to produce phosphoric acid. Write a balanced chemical equation for this reaction. (b) Calcium phosphate reacts with silicon dioxide and carbon graphite at elevated temperatures to produce white phosphorous (P4) as a gas along with calcium silicate (Silcate ion is SiO3²-) and carbon monoxide. Write a balanced chemical equation for this reaction.arrow_forwardProblem Set 4a Chem 1411. A latex balloon is filled with a total of carbon dioxide gas so that its volume reaches 1.352 L. The balloon whose weight was originally 0.753 g, now weighs 2.538 g. How many molecules of carbon dioxide have been added to the balloon?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





