
Concept explainers
a Draw a pH titration curve that represents the titration of 25.0 mL of 0.15 M propionic acid. CH3CH2COOH, by the addition of 0.15 M KOH from a buret. Label the axes and put a scale on each axis. Show where the equivalence point and the buffer region are on the titration curve. You should do calculations for the 0%, 50%, 60%, and 100% titration points. b Is the solution neutral, acidic, or basic at the equivalence point? Why?
(a)
Interpretation:
For titration of 25.0 mL of 0.15 M propionic acid,
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 50%, 60% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.120QP
A pH titration curve showing the equivalence point and buffer region is given in Figure 1 as follows,
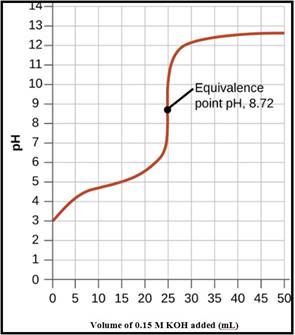
Figure 1
(a)
The pH at the 0% titration point is 2.85
The pH at the 50% titration point is 4.89
The pH at the 60% titration point is 5.06
The pH at the 100% titration point is 8.88
Explanation of Solution
To Draw: A pH titration curve showing the equivalence point and buffer region
The pH titration curve for the titration of 0.15 M propionic acid with 0.15 M
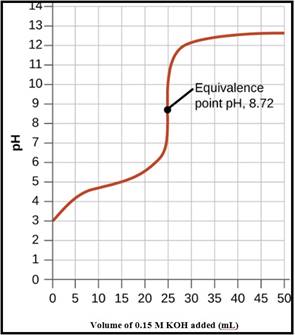
Figure 1
(a)
To Calculate: The pH of the titration points for the 0%, 50 %, 60% and 100%
Given data:
Titration of 0.15 M propionic acid with 0.15 M
pH at the 0% titration point:
Construct an equilibrium table with x as unknown concentration
Consider propionic acid as
|
|
|||
| Initial |
0.15
0.15-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Substitute equilibrium concentrations into the equilibrium-constant equation.
The
Assume x is negligible compared to 0.15 M
Therefore, the concentration of hydronium ion
In the end, pH is calculated as follows,
Therefore, the pH at the 0% titration point is 2.85
pH at the 50% titration point:
The pH is calculated as follows,
Therefore, the pH at the 50% titration point is 4.89
pH at the 60% titration point:
For convenience, express the concentrations as percents.
Substitute the concentrations into the equilibrium expression.
Therefore, the concentration of hydronium ion
In the end, pH is calculated as follows,
Therefore, the pH at the 60% titration point is 5.06
pH at the 100% titration point:
The salt that got produced has undergone a twofold dilution.
Therefore,
Construct an equilibrium table with x as unknown concentration
|
|
|||
| Initial |
0.0750
0.0750-x |
0.00 | 0.00 |
| Change |
|
|
|
| Equilibrium |
x | x | |
Now, calculate
Substitute into the equilibrium constant expression.
Here, x gives the concentration of hydroxide ion,
The pH is calculated as follows,
Therefore, the pH at the 100% titration point is 8.88
The pH at the 0% titration point was calculated as 2.85
The pH at the 50% titration point was calculated as 4.89
The pH at the 60% titration point was calculated as 5.06
The pH at the 100% titration point was calculated as 8.88
(b)
Interpretation:
For titration of 25.0 mL of 0.15 M propionic acid,
A pH titration curve showing the equivalence point and buffer region has to be drawn
- (a) The pH of the titration points for the 0%, 50%, 60% and 100% has to be calculated
- (b) Whether the solution at the equivalence point is neutral, acidic or basic has to be explained
Concept Introduction:
Equivalence point:
The equivalence point in titration is the point where the amount of standard titrant solution (in moles) and the unknown concentration analyte solution (in moles) becomes equal.
In other words, the equivalence point is the point obtained in a titration once a stoichiometric amount of reactant has been added.
Relationship between pH and pOH:
Answer to Problem 16.120QP
The solution at the equivalence point is basic
Explanation of Solution
To Explain: Whether the solution at the equivalence point is neutral, acidic or basic
As a result of titration Potassium propionate salt is produced.
Potassium propionate is the salt of a weak acid and a strong base.
Propionate ion reacts with water to produce hydroxide ions.
Therefore, the given solution is basic
The solution at the equivalence point was found as basic
Want to see more full solutions like this?
Chapter 16 Solutions
General Chemistry
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
- Explain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forwardDraw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forward
- Draw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forwardRecord the IUPAC names for each of the structures shown below. a) b) c) OH d) OH e)arrow_forwardA solution of 14 g of a nonvolatile, nonelectrolyte compound in 0.10 kg of benzene boils at 81.7°C. If the BP of pure benzene is 80.2°C and the K, of benzene is 2.53°C/m, calculate the molar mass of the unknown compound. AT₁ = Km (14)arrow_forward
- Please help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.arrow_forwardDraw the arrow pushing reaction mechanism. DO NOT ANSWER IF YOU WONT DRAW IT. Do not use chat gpt.arrow_forwardComplete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





