Pearson eText for Chemistry: structures and Properties -- Instant Access (Pearson+)
2nd Edition
ISBN: 9780136951537
Author: Nivaldo Tro
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 126E
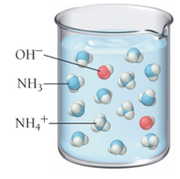
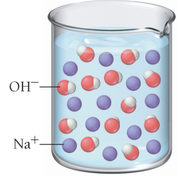
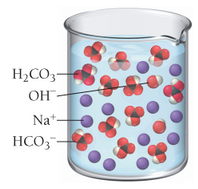
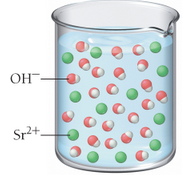
Based on these molecular views, determine whether each pictured base is weak or strong.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3
On the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.
Rank the compounds below from lowest to highest melting point.
Chapter 16 Solutions
Pearson eText for Chemistry: structures and Properties -- Instant Access (Pearson+)
Ch. 16 - In the opening section of this chapter text, we...Ch. 16 - What are the general physical and chemical...Ch. 16 - What is a carboxylic acid? Give an example?Ch. 16 - What is the Arrhenius definition of an acid? Of a...Ch. 16 - What is a hydronium ion? Does H+ exist in solution...Ch. 16 - What is the Bronsted-Lowry definition of an acid?...Ch. 16 - Why is there more than one definition of acid-base...Ch. 16 - Describe amphoteric behavior and give an example.Ch. 16 - What is a conjugate acid-base pair? Provide an...Ch. 16 - Explain the difference between a strong acid and a...
Ch. 16 - For a binary acid, H-Y, which factors affect the...Ch. 16 - Which factors affect the relative acidity of an...Ch. 16 - What are diprotic and triprotic acids? List an...Ch. 16 - Define the acid ionization constant and explain...Ch. 16 - Write an equation for the autoionization of water...Ch. 16 - What happens to the [OH-] of a solution when the...Ch. 16 - Define pH. What pH range is considered acidic?...Ch. 16 - Define pOH. What pOH range is considered acidic?...Ch. 16 - In most solutions containing a strong or weak...Ch. 16 - When calculating [H3O+] for weak acid solutions,...Ch. 16 - What is the percent ionization of an acid? Explain...Ch. 16 - In calculating [H3O+] for a mixture of a strong...Ch. 16 - Write a generic equation showing how a weak base...Ch. 16 - How can you identified if an anion will act as a...Ch. 16 - What is the relationship between the acid...Ch. 16 - What kinds of cations act as weak acids? List some...Ch. 16 - When calculating the [H3O+] for a polyprotic acid,...Ch. 16 - For a weak diprotic acid H2X, what is the...Ch. 16 - Prob. 29ECh. 16 - Prob. 30ECh. 16 - Identify each substance as an acid or a base and...Ch. 16 - Identify each substance as an acid or a base and...Ch. 16 - In each reaction, identify the Bronsted-Lowry...Ch. 16 - In each reaction, identify the Bronsted-Lowry...Ch. 16 - Write the formula for the conjugate base of each...Ch. 16 - Write the formula for the conjugate acid of each...Ch. 16 - Both H2O and H2PO4 are amphoteric. Write an...Ch. 16 - Both HCO3 and HS are amphoteric. Write an equation...Ch. 16 - Prob. 39ECh. 16 - Based on molecular structure, arrange the binary...Ch. 16 - Based on their molecular structure, pick the...Ch. 16 - Based on molecular structure, arrange the oxyacids...Ch. 16 - Prob. 43ECh. 16 - Which is a stronger base, PO43 or AsO43 ? Explain.Ch. 16 - Classify each acid as strong or weak. If the acid...Ch. 16 - Classify each acid as strong or weak. If the acid...Ch. 16 - The three diagrams represent three different...Ch. 16 - Rank the solutions in order of decreasing [H3O+] :...Ch. 16 - Calculate [OH-] in each aqueous solution at 25°C,...Ch. 16 - Calculate [H3O+] in each aqueous solution at 25°C,...Ch. 16 - Calculate the pH and pH of each solution....Ch. 16 - Calculate [H3O+] and [OH-] for each solution. pH =...Ch. 16 - Complete the table. (All solutions are at 25°C.)...Ch. 16 - Prob. 54ECh. 16 - all equilibrium constants, the value of Kw depends...Ch. 16 - The value of KWincreases with temperature. Is the...Ch. 16 - Calculate the pH of each acid solution. Explain...Ch. 16 - Find the concentration of H3O+, to the correct...Ch. 16 - For each strong acid solution, determine [H3O+],...Ch. 16 - Prob. 60ECh. 16 - What mass of HI should be present in 0.250 L of...Ch. 16 - Prob. 62ECh. 16 - What is the pH of solution in which 224 mL of...Ch. 16 - What volume of a concentrated HCl solution, which...Ch. 16 - Determine the [H3O+] and pH of a 0.100 M solution...Ch. 16 - Prob. 66ECh. 16 - Determine the pH of an HNO2 solution of each...Ch. 16 - Determine the pH of an HF solution of each...Ch. 16 - If 15.0 mL of glacial acetic acid (pure HC2H3O2)...Ch. 16 - Calculate the pH of a formic acid solution that...Ch. 16 - A 0.185 M solution of a weak acid (HA) has a pH of...Ch. 16 - Prob. 72ECh. 16 - Determine the percent ionization of a 0.125 M HCN...Ch. 16 - Determine the percent ionization of a 0.225 M...Ch. 16 - Calculate the percent ionization of an acetic acid...Ch. 16 - Calculate the percent ionization of a formic acid...Ch. 16 - A 0.148 M solution of a monoprotic acid has a...Ch. 16 - Prob. 78ECh. 16 - Prob. 79ECh. 16 - Prob. 80ECh. 16 - Find the pH of each mixture of acids. 0.115 M in...Ch. 16 - Find the pH of each mixture of acids. 0.075 M in...Ch. 16 - For each strong base solution, determine [OH-],...Ch. 16 - Prob. 84ECh. 16 - Prob. 85ECh. 16 - Prob. 86ECh. 16 - Prob. 87ECh. 16 - Prob. 88ECh. 16 - Prob. 89ECh. 16 - Prob. 90ECh. 16 - Determine the [OH-], pH, and pOH of a 0.15 ammonia...Ch. 16 - Determine the [OH-], pH, and pOH of a solution...Ch. 16 - Prob. 93ECh. 16 - Prob. 94ECh. 16 - Prob. 95ECh. 16 - Prob. 96ECh. 16 - Determine if each anion is basic or neutral. For...Ch. 16 - Determine whether each anion is basic or neutral....Ch. 16 - Prob. 99ECh. 16 - Determine the [OH-] and pH of a solution is 0.250...Ch. 16 - Determine whether each cation is acidic or...Ch. 16 - Prob. 102ECh. 16 - Determine if each salt will form a solution that...Ch. 16 - Prob. 104ECh. 16 - Prob. 105ECh. 16 - Prob. 106ECh. 16 - Prob. 107ECh. 16 - Prob. 108ECh. 16 - Prob. 109ECh. 16 - Prob. 110ECh. 16 - Prob. 111ECh. 16 - Prob. 112ECh. 16 - Write chemical equations and corresponding...Ch. 16 - Prob. 114ECh. 16 - Prob. 115ECh. 16 - Calculate the [H3O+] and pH of each polyprotic...Ch. 16 - Calculate the concentration of each species in a...Ch. 16 - Calculate the concentration of each species in a...Ch. 16 - Calculate the [H3O+] and pH of each H2S04...Ch. 16 - Consider a 0.10 M solution of a weak polyprotic...Ch. 16 - Classify each species as a Lewis acid or a Lewis...Ch. 16 - Prob. 122ECh. 16 - Prob. 123ECh. 16 - Prob. 124ECh. 16 - Prob. 125ECh. 16 - Based on these molecular views, determine whether...Ch. 16 - The binding of oxygen by hemoglobin in the blood...Ch. 16 - Carbon dioxide dissolves in water according to the...Ch. 16 - People often take Milk of Magnesia to reduce the...Ch. 16 - Lakes that have been acidified by acid rain (which...Ch. 16 - Acid rain over the Great lakes has a pH of about...Ch. 16 - White wines tend to be more acidic than red wines....Ch. 16 - Common aspirin is acetylsalicylic acid, which has...Ch. 16 - The AIDS drug zalcitabine (also known as ddC) is a...Ch. 16 - Determine the pH of each solution. 0.0100MHCIO4...Ch. 16 - Determine the pH of each solution. 0.0650M HNO3...Ch. 16 - Determine the pH of each two-component solution....Ch. 16 - Determine the pH of each two-component solution....Ch. 16 - Write net ionic equations for the reactions that...Ch. 16 - Prob. 140ECh. 16 - The pH of a 1.00 M solution of urea, a weak...Ch. 16 - Prob. 142ECh. 16 - Lactic acid is a weak acid found in milk. Its...Ch. 16 - Prob. 144ECh. 16 - A student mistakenly calculates the pH of a 1.0107...Ch. 16 - When 2.55 g of an unknown weak acid (HA) with a...Ch. 16 - Prob. 147ECh. 16 - To what volume should you dilute 1 L of a solution...Ch. 16 - HA, a weak acid, with Ka=1.0108 , also forms the...Ch. 16 - Prob. 150ECh. 16 - Prob. 151ECh. 16 - To 1.0 L of a 0.30 M solution of HCIO2 is added...Ch. 16 - A mixture of Na2CO3 and NaHCO3 has a mass of 82.2...Ch. 16 - Prob. 154ECh. 16 - Prob. 155ECh. 16 - Prob. 156ECh. 16 - Prob. 157ECh. 16 - Prob. 158ECh. 16 - Without referring to the text, go around your...Ch. 16 - Prob. 160ECh. 16 - Prob. 161ECh. 16 - Prob. 162ECh. 16 - Prob. 163ECh. 16 - Prob. 164ECh. 16 - Identify the conjugate base in the reaction shown...Ch. 16 - Which pair is a Bronsted-Lowry conjugate acid-base...Ch. 16 - Which acid has the largest Ka: HClO2(aq),...Ch. 16 - Consider the given acid ionization constants and...Ch. 16 - What is the OH- concentration in an aqueous...Ch. 16 - An HNO3(aq) solution has a pH of 1.75. What is the...Ch. 16 - Find the pH of a 0.350 M aqueous benzoic acid...Ch. 16 - Find the pH of a 0.155 M HClO2(aq) solution. For...Ch. 16 - 9. Calculate the percent ionization of 1.45 M...Ch. 16 - Consider two aqueous solutions of nitrous acid...Ch. 16 - What is the [OH-] in a 0.200 M solution of...Ch. 16 - Which ion will be basic in aqueous solution? HSO4-...Ch. 16 - Which compound will form an acidic solution when...Ch. 16 - Find the pH of 0.175 M NaCN solution. For HCN,...Ch. 16 - What is the concentration of X2- in a 0.150 M...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forwardsomeone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forward
- The dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardSuppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forward
- What mass of ethylene glycol, HOCH2CH2OH, Mustbe added to 5.50 kg of water to antifreeze that would work for the car radiator to -10.0 degrees celcius? MM (g/mol): 62.07arrow_forwardWhat is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/molarrow_forwardThe rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?arrow_forward
- Handwritten pleasearrow_forwardChoose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY