
Concept explainers
Draw structures corresponding to the following names:
(a) 3-Methyl-1, 2-benzenediamine
(b) 1, 3, 5-Benzenetriol
(c) 3-Methyl-2-phenylhexane
(d) o-Aminobenzoic acid
(e) m-Bromophenol
(f) 2, 4, 6-Trinitrophenol (picric acid]
a) 3-methyl-1,2-benzenediamine
Interpretation:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine.
Answer to Problem 19AP
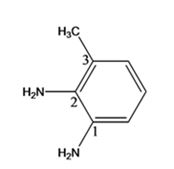
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine is
Explanation of Solution
The IUPAC name of the compound, 3-methyl-1,2-benzenediamine, indicates that the compound has a benzene ring with a methyl group on C3 and two amino groups on C1 & C2.
The structure of the compound with IUPAC name 3-methyl-1,2-benzenediamine

b) 1,3,5-benzenetriol
Interpretation:
The structure of the compound with IUPAC name 1,3,5-benzenetriol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment as carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 1,3,5-benzenetriol.
Answer to Problem 19AP
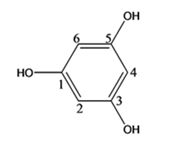
The structure of the compound with IUPAC name 1,3,5-benzenetriol is
Explanation of Solution
The IUPAC name of the compound, 1,3,5-benzenetriol, indicates that the compound has a benzene ring with three hydroxyl groups on C1, C3 & C5.
The structure of the compound with IUPAC name 1,3,5-benzenetriol is

c) 3-methyl-2-phenylhexane
Interpretation:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is to be given.
Concept introduction:
Alkyl-substituted benzenes, called arenes, are named in different ways depending on the size of the alkyl group. If the alkyl group is smaller than the ring (six or fewer carbons), the arene is named as an alkyl-substituted benzene. If the alkyl substituent is larger than the ring (seven or more carbons), the arene is named as a phenyl-substitited alkane.
To draw:
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane.
Answer to Problem 19AP
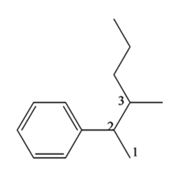
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is
Explanation of Solution
The IUPAC name of the compound, 3-methyl-2-phenyl hexane, indicates that the compound has a six carbon straight chain with a phenyl group on C2 and a methyl group on C3.
The structure of the compound with IUPAC name 3-methyl-2-phenylhexane is

d) o-aminobenzoic acid
Interpretation:
The structure of the compound with IUPAC name o-aminobenzoic acid is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta(m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name p-aminobenzoic acid.
Answer to Problem 19AP
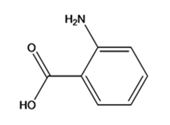
The structure of the compound with IUPAC name p-aminobenzoic acid is
Explanation of Solution
The IUPAC name of the compound, p-aminobenzoic acid, indicates that the compound has a benzene ring with a carboxyl and amino groups in 1,2-relationship.
The structure of the compound with IUPAC name p-aminobenzoicacid is

e) m-bromophenol
Interpretation:
The structure of the compound with IUPAC name m-bromophenol is to be given.
Concept introduction:
Disubstituted benzenes are named using the prefixes ortho (o), meta (m) and para (p). An ortho-disubstituted benzene has its two substituent groups in a 1,2- relationship on the ring. A meta-disubstituted benzene has its two substituent groups in a 1,3-relationship on the ring. A para-disubstituted benzene has its two substituent groups in a 1,4-relationship on the ring. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name m-bromophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name m-bromophenol is
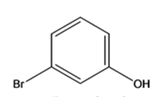
Explanation of Solution
The IUPAC name of the compound, m-bromophenol, indicates that the compound has a benzene ring with a hydroxyl group and bromine atom in 1,3-relationship.
The structure of the compound with IUPAC name m-bromophenol is

f) 2,4,6-trinitrophenol
Interpretation:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is to be given.
Concept introduction:
Benzene with more than two substituent groups are named choosing a point of attachment ac carbon 1 and numbering the substituent groups on the ring so that the second substituent has as low number as possible. If ambiguity still exists, numbering is done such that the third and fourth substituent groups have a number as low as possible, until a point of difference is obtained. While writing the name the substituent groups are arranged alphabetically.
To draw:
The structure of the compound with IUPAC name 2,4,6-trinitrophenol.
Answer to Problem 19AP
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is
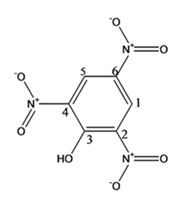
Explanation of Solution
The IUPAC name of the compound, 2,4,6-trinitrophenol, indicates that the compound has a benzenering with a hydroxyl group on C1 and three nitro groups on C2, C4 & C6.
The structure of the compound with IUPAC name 2,4,6-trinitrophenol is

Want to see more full solutions like this?
Chapter 15 Solutions
EBK ORGANIC CHEMISTRY
- Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forward
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





