
CHEMISTRY:ATOMS FIRST-2 YEAR CONNECT
2nd Edition
ISBN: 9781260592320
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15.4, Problem 7PPC
Consider the reaction
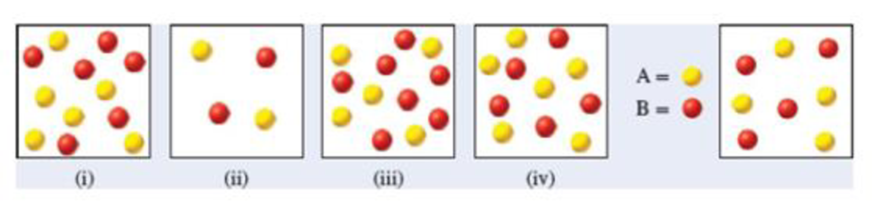
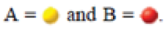 For each of the following diagrams [(i)-(iv)], indicate whether the reaction will proceed to the right, the left, or neither to achieve equilibrium.
For each of the following diagrams [(i)-(iv)], indicate whether the reaction will proceed to the right, the left, or neither to achieve equilibrium.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 15 Solutions
CHEMISTRY:ATOMS FIRST-2 YEAR CONNECT
Ch. 15.2 - Write reaction quotients for the following...Ch. 15.2 - Write the reaction quotient for each of the...Ch. 15.2 - Write the equation for the equilibrium that...Ch. 15.2 - In principle, in the reaction of A and B to form...Ch. 15.2 - Carbonyl chloride (COCl2), also called phosgene,...Ch. 15.2 - In an analysis of the following reaction at 100C....Ch. 15.2 - In another analysis at 100C involving the same...Ch. 15.2 - Consider the reaction 2AB. The diagram shown on...Ch. 15.2 - Prob. 15.2.1SRCh. 15.2 - Prob. 15.2.2SR
Ch. 15.3 - Write equilibrium expressions for each of the...Ch. 15.3 - Write equilibrium expressions for each of the...Ch. 15.3 - Which of the following equilibrium expressions...Ch. 15.3 - Consider the reaction A(s)+B(g)C(s). Which of the...Ch. 15.3 - The following reactions have the indicated...Ch. 15.3 - The following reactions have the indicated...Ch. 15.3 - Using the data from Practice Problem A, determine...Ch. 15.3 - Consider a chemical reaction represented by the...Ch. 15.3 - Write KP expressions for (a) PCl3(g) + Cl2(g) ...Ch. 15.3 - Write KP expressions for...Ch. 15.3 - Write the equation for the gaseous equilibrium...Ch. 15.3 - These diagrams represent closed systems at...Ch. 15.3 - The equilibrium constant, Kc, for the reaction...Ch. 15.3 - For the reaction N2(g)+3H2(g)2NH2(g) KC is 2.3 ...Ch. 15.3 - KP = 2.79 10-5 for the reaction in Practice...Ch. 15.3 - Consider the reaction 2A(l)2B(g) at room...Ch. 15.3 - Prob. 15.3.1SRCh. 15.3 - Prob. 15.3.2SRCh. 15.3 - Prob. 15.3.3SRCh. 15.3 - Prob. 15.3.4SRCh. 15.4 - At 375C, the equilibrium constant for the reaction...Ch. 15.4 - The equilibrium constant, Kc, for the formation of...Ch. 15.4 - Calculate KP for the formation of nitrosyl...Ch. 15.4 - Consider the reaction 2AB. The diagram shown on...Ch. 15.4 - The equilibrium constant, KP, for the reaction...Ch. 15.4 - G for the reaction H2(g)+I2(s)2HI(g) is 2.60...Ch. 15.4 - Prob. 8PPBCh. 15.4 - Prob. 8PPCCh. 15.4 - Using data from Appendix 2, calculate the...Ch. 15.4 - Prob. 9PPACh. 15.4 - Kf for the complex ion Ag(NH3)2+ is 1.5 107 at...Ch. 15.4 - Which of the following graphs [(i)(iv)] best shows...Ch. 15.4 - The equilibrium constant, Ksp, for the dissolution...Ch. 15.4 - Calculate G for the process:...Ch. 15.4 - Ksp for Co(OH)2 at 25C is 3.3 10-16 Using this and...Ch. 15.4 - Prob. 10PPCCh. 15.4 - Prob. 15.4.1SRCh. 15.4 - Prob. 15.4.2SRCh. 15.4 - Prob. 15.4.3SRCh. 15.5 - Kc for the reaction of hydrogen and iodine to...Ch. 15.5 - Calculate the equilibrium concentrations of H2,...Ch. 15.5 - Determine the initial concentration of HI if the...Ch. 15.5 - Consider the reaction A(g) + B(g) C(g). The...Ch. 15.5 - For the same reaction and temperature as in Worked...Ch. 15.5 - Prob. 12PPACh. 15.5 - Prob. 12PPBCh. 15.5 - Prob. 12PPCCh. 15.5 - At elevated temperatures, iodine molecules break...Ch. 15.5 - Aqueous hydrocyanic acid (HCN) ionizes according...Ch. 15.5 - Consider a weak acid, HA, that ionizes according...Ch. 15.5 - Prob. 13PPCCh. 15.5 - A mixture of 5.75 atm of H2 and 5.75 atm of I2 is...Ch. 15.5 - Prob. 14PPACh. 15.5 - Prob. 14PPBCh. 15.5 - Consider the reaction A(g)+B(g)C(s)+D(s). The...Ch. 15.5 - Prob. 15.5.1SRCh. 15.5 - Prob. 15.5.2SRCh. 15.5 - Prob. 15.5.3SRCh. 15.6 - Hydrogen sulfide (H2S) is a contaminant commonly...Ch. 15.6 - For each change indicated, determine whether the...Ch. 15.6 - What can be added to the equilibrium that will (a)...Ch. 15.6 - Consider the reaction A(g)+B(g)C(s)+D(s), of the...Ch. 15.6 - For each reaction, predict in what direction the...Ch. 15.6 - For each reaction, predict the direction of shift...Ch. 15.6 - For the following equilibrium, give an example of...Ch. 15.6 - Prob. 16PPCCh. 15.6 - Prob. 15.6.1SRCh. 15.6 - Prob. 15.6.2SRCh. 15.6 - Prob. 15.6.3SRCh. 15.6 - Prob. 15.6.4SRCh. 15 - Define equilibrium. Give two examples of a dynamic...Ch. 15 - Which of the following statements is collect about...Ch. 15 - Consider the reversible reaction A B. Explain how...Ch. 15 - What is the law of mass action?Ch. 15 - Briefly describe the importance of equilibrium in...Ch. 15 - Define reaction quotient. How does it differ from...Ch. 15 - Prob. 15.7QPCh. 15 - Write the equation for the reaction that...Ch. 15 - Prob. 15.9QPCh. 15 - Prob. 15.10QPCh. 15 - Prob. 15.11QPCh. 15 - The equilibrium constant for the reaction...Ch. 15 - Prob. 15.13QPCh. 15 - Prob. 15.14QPCh. 15 - Prob. 15.15QPCh. 15 - Prob. 15.16QPCh. 15 - Prob. 15.17QPCh. 15 - Write equilibrium constant expressions for Kc and...Ch. 15 - Write the equilibrium constant expressions for Kc...Ch. 15 - Prob. 15.20QPCh. 15 - Prob. 15.21QPCh. 15 - Prob. 15.22QPCh. 15 - Computational Problems 15.23 The equilibrium...Ch. 15 - Prob. 15.24QPCh. 15 - The equilibrium constant KP for the reaction is...Ch. 15 - Prob. 15.26QPCh. 15 - Prob. 15.27QPCh. 15 - Prob. 15.28QPCh. 15 - Prob. 15.29QPCh. 15 - The equilibrium constant Kp for foe reaction is...Ch. 15 - Ammonium carbamate (NH4CO2NH2) decomposes as...Ch. 15 - Prob. 15.32QPCh. 15 - Consider the equilibrium If nitrosyl bromide...Ch. 15 - Prob. 15.34QPCh. 15 - The following equilibrium constants have been...Ch. 15 - The following equilibrium constants were...Ch. 15 - At a certain temperature, the following reactions...Ch. 15 - Prob. 15.38QPCh. 15 - The equilibrium constant for the reaction A B is...Ch. 15 - Prob. 15.40QPCh. 15 - Explain why Equation 15.6 is of great importance...Ch. 15 - Fill in the missing entries in the following...Ch. 15 - Computational Problems 15.43 The aqueous reaction...Ch. 15 - For the autoionization of water at 25C,...Ch. 15 - Consider the following reaction at 25C....Ch. 15 - Prob. 15.46QPCh. 15 - (a) Calculate G and KP for the following...Ch. 15 - The equilibrium constant (KP) for the reaction...Ch. 15 - Consider the decomposition of calcium carbonate....Ch. 15 - The equilibrium constant KP for the reaction CO(g)...Ch. 15 - Prob. 15.51QPCh. 15 - Prob. 15.52QPCh. 15 - Prob. 15.53QPCh. 15 - Conceptual Problems 15.54 A and B react to form...Ch. 15 - If Kc. = 2 for the reaction A2 + B2 2AB at a...Ch. 15 - Prob. 15.1VCCh. 15 - Prob. 15.2VCCh. 15 - Prob. 15.3VCCh. 15 - Prob. 15.4VCCh. 15 - Review Questions Outline the steps for calculating...Ch. 15 - Prob. 15.57QPCh. 15 - Prob. 15.58QPCh. 15 - Prob. 15.59QPCh. 15 - The dissociation of molecular iodine into iodine...Ch. 15 - The equilibrium constant Kc for the decomposition...Ch. 15 - Consider the following equilibrium process at...Ch. 15 - Prob. 15.63QPCh. 15 - Prob. 15.64QPCh. 15 - Prob. 15.5VCCh. 15 - Prob. 15.6VCCh. 15 - Prob. 15.7VCCh. 15 - Prob. 15.8VCCh. 15 - Prob. 15.9VCCh. 15 - Prob. 15.10VCCh. 15 - Prob. 15.11VCCh. 15 - Prob. 15.12VCCh. 15 - Prob. 15.65QPCh. 15 - Prob. 15.66QPCh. 15 - Prob. 15.67QPCh. 15 - Conceptual Problems 15.68 Which of the following...Ch. 15 - For which of the following reactions will a change...Ch. 15 - Which of the following equilibria will shift to...Ch. 15 - Which of the following will cause the equilibrium...Ch. 15 - Consider the following equilibrium system...Ch. 15 - Heating solid sodium bicarbonate in a closed...Ch. 15 - Consider the following equilibrium systems....Ch. 15 - What effect does an increase in pressure have on...Ch. 15 - Prob. 15.76QPCh. 15 - Consider the following equilibrium process....Ch. 15 - Prob. 15.78QPCh. 15 - Consider the following equilibrium reaction in a...Ch. 15 - Consider the gas-phase reaction...Ch. 15 - Prob. 15.81QPCh. 15 - Prob. 15.82QPCh. 15 - Prob. 15.83QPCh. 15 - The simplified equation representing the binding...Ch. 15 - Prob. 15.85QPCh. 15 - ADDITIONAL PROBLEMS 15.86 Consider the following...Ch. 15 - The equilibrium constant Kp for the reaction...Ch. 15 - For a reaction with a negative G value, which of...Ch. 15 - Carbon monoxide (CO) and nitric oxide (NO) are...Ch. 15 - Consider the following reacting system....Ch. 15 - At a certain temperature and a total pressure of...Ch. 15 - The decomposition of ammonium hydrogen sulfide...Ch. 15 - Consider the reaction 2NO(g)+O2(g)2NO2(g) At 430C,...Ch. 15 - In the Mond process for the purification of...Ch. 15 - Consider the reaction N2(g)+O2(g)2NO(g) Given that...Ch. 15 - Prob. 15.96QPCh. 15 - A mixture of 0.47 mole of H2 and 3.59 moles of HCl...Ch. 15 - Prob. 15.98QPCh. 15 - The following reaction represents the removal of...Ch. 15 - Prob. 15.100QPCh. 15 - Prob. 15.101QPCh. 15 - Calculate the equilibrium pressure of CO2 due to...Ch. 15 - Prob. 15.103QPCh. 15 - Consider the gas-phase reaction between A2 (green)...Ch. 15 - Prob. 15.105QPCh. 15 - The following diagram represents a gas-phase...Ch. 15 - The formation of SO3 from SO2 and O2 is an...Ch. 15 - Calculate the pressure of O2 (in atm) over a...Ch. 15 - The following reaction was described as the cause...Ch. 15 - Prob. 15.110QPCh. 15 - Calculate G and Kp for the following processes at...Ch. 15 - Prob. 15.112QPCh. 15 - The equilibrium constant Kp for the following...Ch. 15 - Prob. 15.114QPCh. 15 - Prob. 15.115QPCh. 15 - Prob. 15.116QPCh. 15 - Prob. 15.117QPCh. 15 - Prob. 15.118QPCh. 15 - Prob. 15.119QPCh. 15 - Prob. 15.120QPCh. 15 - The equilibrium constant Kc for the reaction...Ch. 15 - For reactions earned out under standard-state...Ch. 15 - When a gas was heated under atmospheric...Ch. 15 - Prob. 15.124QPCh. 15 - The equilibrium constant Kc for the following...Ch. 15 - The equilibrium constant (KP for the formation of...Ch. 15 - Prob. 15.127QPCh. 15 - Prob. 15.128QPCh. 15 - Prob. 15.129QPCh. 15 - In the gas phase, nitrogen dioxide is actually a...Ch. 15 - A 2.50-mole sample of NOCl was initially in a...Ch. 15 - About 75% of hydrogen for industrial use is...Ch. 15 - Photosynthesis can be represented by...Ch. 15 - Consider the decomposition of ammonium chloride at...Ch. 15 - At 25C, the equilibrium partial pressures of NO2...Ch. 15 - In 1899 the German chemist Ludwig Mond developed a...Ch. 15 - Consider the equilibrium reaction described in...Ch. 15 - Consider the equilibrium system3AB. Sketch the...Ch. 15 - The vapor pressure of mercury is 0.0020 mmHg at...Ch. 15 - Large quantities of hydrogen are needed for the...Ch. 15 - Prob. 15.141QPCh. 15 - At 25C. a mixture of NO2 and N2O4 gases are m...Ch. 15 - Prob. 15.143QPCh. 15 - Heating copper (II) oxide at 400C does not produce...Ch. 15 - The equilibrium constant Kc for the reaction...Ch. 15 - The dependence of the equilibrium constant of a...Ch. 15 - Prob. 15.147QPCh. 15 - The following diagram shows the variation of the...Ch. 15 - The Kp for the reaction SO2Cl2(g)SO2(g)+Cl2(g) is...Ch. 15 - Derive the equation G=RTlnQK where Q is the...Ch. 15 - Prob. 15.151QPCh. 15 - Prob. 15.152QPCh. 15 - Prob. 15.153QPCh. 15 - Industrial production of ammonia from hydrogen and...Ch. 15 - For which of the following reactions is Kc equal...Ch. 15 - At present, the World Anti-Doping Agency has no...Ch. 15 - (a) Use the vant Hoff equation in Problem 15.146...Ch. 15 - The Ka for hydrocyanic acid (HCN) is 4.9 10 l0....Ch. 15 - Determine the concentrations of Pb2+ and I in a...Ch. 15 - Determine the Ka for a weak acid if a 0.10-M...Ch. 15 - Prob. 15.4KSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY