
Interpretation:
Hydrogenation hydrolysis and saponification are important reaction of hydrocarbons.
(i) Hydrogenation reaction it is addition reaction of atom to unsaturated carbon
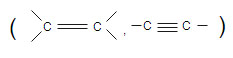
In the presence metal catalyst . In this reaction saturated hydrocarbon are obtained from
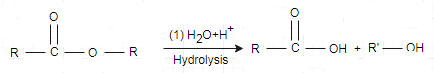
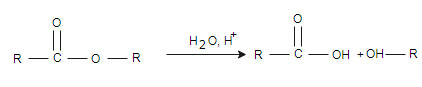
(ii) Hydrolysis: hydrolysis reaction is just reverse of esterification. In hydrolysis one water molecule is added and split the ester. This reaction is done in the presence strong acid or digestive enzymes like
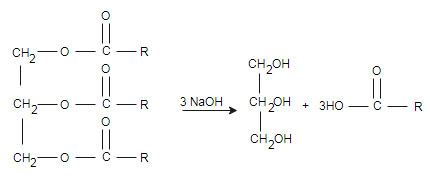
(iii) Saponification: saponification is a reaction used in the formation of soap
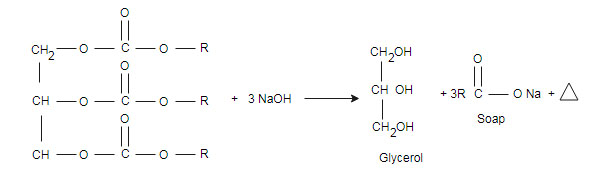
Concept used:
(i) Hydrogenation reaction
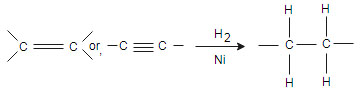
(ii) Hydrolysis:
(iii) Saponification:
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry, Books a la Carte Edition & Modified MasteringChemistry with Pearson eText -- ValuePack Access Card Package
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





