
CHEMISTRY >CUSTOM<
14th Edition
ISBN: 9781259137815
Author: Julia Burdge
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 82AP
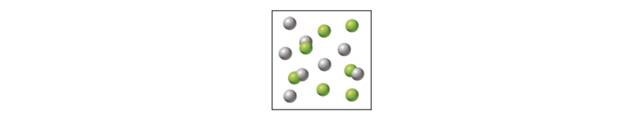
The following diagram represents a gas-phase equilibrium mixture for the reaction
temperature. Describe what would happen to the system after each of the following changes: (a) the temperature is decreased, (b) the volume is increased, (c) He atoms are added to the mixture at constant volume, (d) a catalyst is added to the mixture.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a mental model for calcium chloride mixed with sodium phosphate
here is my question (problem number 20) please explain to me thanks!
The bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).
Chapter 15 Solutions
CHEMISTRY >CUSTOM<
Ch. 15.1 - Practice Problem ATTEMPT
In an analysis of the...Ch. 15.1 - Prob. 1PPBCh. 15.1 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 15.2 - Practice ProblemATTEMPT Write the reaction...Ch. 15.2 - Practice Problem BUILD
Write the equation for the...Ch. 15.2 - Practice ProblemCONCEPTUALIZE In principle, in the...Ch. 15.2 - Select the correct equilibrium expression for the...Ch. 15.2 - Prob. 2CPCh. 15.3 - Practice Problem ATTEMPT Write equilibrium...Ch. 15.3 - Practice Problem BUILD
Which of the following...
Ch. 15.3 - Prob. 1PPCCh. 15.3 - Prob. 1CPCh. 15.3 - Prob. 2CPCh. 15.3 - Given the following information: HF ( a q ) ⇄ H +...Ch. 15.3 - Prob. 4CPCh. 15.4 - Practice ProblemATTEMPT The following reactions...Ch. 15.4 - Practice Problem BUILD
The equation represents a...Ch. 15.4 - Practice ProblemCONCEPTUALIZE Consider a chemical...Ch. 15.4 - Use the following information to answer questions...Ch. 15.4 - Prob. 2CPCh. 15.4 - 15.4.3 If for the reaction at a certain...Ch. 15.4 - If K c = 3 for the reaction X + 2Y ⇄ Z at a...Ch. 15.5 - Practice ProblemATTEMPT Write K? expressions for (...Ch. 15.5 - Prob. 1PPBCh. 15.5 - Prob. 1PPCCh. 15.5 - Prob. 1CPCh. 15.5 - Prob. 2CPCh. 15.5 - Prob. 3CPCh. 15.5 - Prob. 4CPCh. 15.5 - Prob. 5CPCh. 15.5 - Prob. 6CPCh. 15.6 - Practice Problem ATTEMPT
For the reaction:
....Ch. 15.6 - Practice ProblemBUILD K p = 2.79 × 10 − 5 for the...Ch. 15.6 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 15.7 - Prob. 1PPACh. 15.7 - Prob. 1PPBCh. 15.7 - Prob. 1PPCCh. 15.8 - Practice ProblemATTEMPT Calculate the equilibrium...Ch. 15.8 - Practice ProblemBUILD Determine the initial...Ch. 15.8 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 15.9 - Prob. 1PPACh. 15.9 - Prob. 1PPBCh. 15.9 - Prob. 1PPCCh. 15.10 - Practice ProblemATTEMPT Aqueous hydrocyanic acid...Ch. 15.10 - Practice Problem BUILD Consider a weak acid, HA,...Ch. 15.10 - Practice ProblemCONCEPTUALIZE Each of the...Ch. 15.11 - Practice Problem ATTEMPT Determine the equilibrium...Ch. 15.11 - Prob. 1PPBCh. 15.11 - Prob. 1PPCCh. 15.12 - Practice ProblemATTEMPT For each change indicated,...Ch. 15.12 - Prob. 1PPBCh. 15.12 - Practice ProblemCONCEPTUALIZE Consider the...Ch. 15.13 - Practice Problem ATTEMPT
For each reaction,...Ch. 15.13 - Practice Problem BUILD
For the following...Ch. 15.13 - Practice Problem CONCEPTUALIZE
Consider the...Ch. 15.14 - Practice Problem ATTEMPT
The reaction of carbon...Ch. 15.14 - Practice Problem BUILD
Consider the hypothetical...Ch. 15.14 - Practice Problem CONCEPTUALIZE
The decomposition...Ch. 15 - The K a for hydrocyanic acid ( HCN ) is 4 .9 × 10...Ch. 15 - 15.2
Determine the concentrations of in a...Ch. 15 - 15.3
Determine the for a weak acid if a 0.10-M...Ch. 15 - Prob. 4KSPCh. 15 - Define equilibrium. Give two examples of a dynamic...Ch. 15 - 15.2 Which of the following statements is correct...Ch. 15 - 15.3 Consider the reversible reaction Explain how...Ch. 15 - What is the law of mass action?Ch. 15 - Briefly describe the importance of equilibrium in...Ch. 15 - Define reaction quotient. How does it differ from...Ch. 15 - Write reaction quotients for the following...Ch. 15 - Write the equation for the reaction that...Ch. 15 - Consider the reaction: 2NO ( g ) + 2H 2 ( g ) ⇄ N...Ch. 15 - The equilibrium constant for the reaction: 2SO 2 (...Ch. 15 - Consider the following equilibrium process at...Ch. 15 - The equilibrium constant for the reaction: 2 H 2 (...Ch. 15 - The first diagram represents a system at...Ch. 15 - Prob. 14QPCh. 15 - Define homogeneous equilibrium and heterogeneous...Ch. 15 - What do the symbols K c and K p represent?Ch. 15 - Write the expressions for the equilibrium...Ch. 15 - Write equilibrium constant expressions for K c ,...Ch. 15 - Write the equilibrium constant expressions for K c...Ch. 15 - 15.20 Write the equation relating to , and define...Ch. 15 - 15.21 The equilibrium constant () for the...Ch. 15 - What is K p at 1273°C for the reaction 2CO ( g ) +...Ch. 15 - 15.23 The equilibrium constant for the...Ch. 15 - 15.24 Consider the reaction:
If the equilibrium...Ch. 15 - 15.25 A reaction vessel contains at equilibrium...Ch. 15 - 15.26 The equilibrium constant Kc for the...Ch. 15 - At equilibrium, the pressure of the reacting...Ch. 15 - The equilibrium constant K p for the reaction: PCl...Ch. 15 - Ammonium carbamate ( NH 4 CO 2 NH 2 ) decomposes...Ch. 15 - The following equilibrium constants were...Ch. 15 - 15.31 At a certain temperature, the following...Ch. 15 - 15.32 Pure phosgene gas , was placed in a 1.50-L...Ch. 15 - Consider the equilibrium: 2 NOBr( g ) ⇄ 2 NO( g...Ch. 15 - The following equilibrium constants have been...Ch. 15 - 15.35 The following equilibrium constants have...Ch. 15 - 15.36 The equilibrium constant for the reaction at...Ch. 15 - The following diagrams represent the equilibrium...Ch. 15 - 15.38 Outline the steps for calculating the...Ch. 15 - 15.39 The equilibrium constant K? for the...Ch. 15 - 15.40 For the synthesis of ammonia:
the...Ch. 15 - For the reaction: H 2 ( g ) + CO 2 ( g ) ⇄ H 2 O (...Ch. 15 - At 1000 K, a sample of pure NO, gas decomposes:...Ch. 15 - The equilibrium constant K c for the reaction H 2...Ch. 15 - The dissociation of molecular iodine into iodine...Ch. 15 - The equilibrium constant Kc for the decomposition...Ch. 15 - 15.46 Consider the following equilibrium process...Ch. 15 - 15.47 Consider the heterogeneous equilibrium...Ch. 15 - The equilibrium constant K c for the reaction: H 2...Ch. 15 - The aqueous reaction: L-glutamate + pyruvate ⇄...Ch. 15 - 15.50 Explain Le Châtelier’s principle. How does...Ch. 15 - Use Le Chatelier's principle to explain why the...Ch. 15 - 15.52 List four factors that can shift the...Ch. 15 - Does the addition of a catalyst have any effects...Ch. 15 - 15.54 Consider the following equilibrium system...Ch. 15 - 15.55 Heating solid sodium bicarbonate in a closed...Ch. 15 - 15.56 Consider the following equilibrium...Ch. 15 - 15.57 What effect does an increase in pressure...Ch. 15 - Prob. 58QPCh. 15 - Consider the following equilibrium process: PCl 5...Ch. 15 - Consider the reaction: 2SO 2 ( g ) ⇄ 2 SO 3 ( g )...Ch. 15 - In the uncatalyzed reaction: N 2 O 4 ( g ) ⇄ 2 NO...Ch. 15 - 15.62 Consider the gas-phase reaction:
Predict...Ch. 15 - Consider the following equilibrium reaction in a...Ch. 15 - 15.64 The following diagrams show the reaction at...Ch. 15 - 15.65 The following diagrams show an equilibrium...Ch. 15 - 15.66 Consider the reaction . The first diagram...Ch. 15 - Prob. 67APCh. 15 - Consider the equilibrium system 3A → B . Sketch...Ch. 15 - Baking soda (sodium bicarbonate) undergoes thermal...Ch. 15 - Consider the following reaction at equilibrium: A...Ch. 15 - Prob. 71APCh. 15 - 15.72 Consider the following reacting...Ch. 15 - 15.73 At a certain temperature and a total...Ch. 15 - The decomposition of ammonium hydrogen sulfide: N...Ch. 15 - 15.75 Consider the following reaction at a certain...Ch. 15 - When heated, ammonium carbamate decomposes as...Ch. 15 - A mixture of 0 .47 mole of H2 and 3 .59 moles of...Ch. 15 - When heated at high temperatures, iodine vapor...Ch. 15 - 15.79 One mole of and three moles of are placed...Ch. 15 - At 1130°C , the equilibrium constant ( K c ) for...Ch. 15 - For the purpose of determining K p using Equation...Ch. 15 - The following diagram represents a gas-phase...Ch. 15 - 15.83 Consider the following reaction at
When...Ch. 15 - 15.84 A quantity of 0.20 mole of carbon dioxide...Ch. 15 - 15.85 When dissolved in water, glucose (com sugar)...Ch. 15 - 15 86 At room temperature, solid iodine is in...Ch. 15 - 15.87 A student placed a few ice cubes in a...Ch. 15 - 15.88 A mixture containing 3.9 moles of and 0.88...Ch. 15 - 15.89 The equilibrium constant for the...Ch. 15 - When heated, a gaseous compound A dissociates as...Ch. 15 - 15.91 When a gas was heated under atmospheric...Ch. 15 - Prob. 92APCh. 15 - A sealed glass bulb contains a mixture of NO 2 and...Ch. 15 - At 20°C , the vapor pressure of water is 0.0231...Ch. 15 - A 2.50-mol sample of NOCl was initially in a...Ch. 15 - 15.96 About 75 percent of hydrogen for industrial...Ch. 15 - Water is a very weak electrolyte that undergoes...Ch. 15 - 15.98 Consider the following reaction, which takes...Ch. 15 - The equilibrium constant Kc for the reaction: 2NH...Ch. 15 - At 25°C, a mixture of NO 2 and N 2 O 4 gases are...Ch. 15 - 15.101 Consider the reaction between and in a...Ch. 15 - In 1899 the German chemist Ludwig Mond developed a...Ch. 15 - For which of the following reactions is K c equal...Ch. 15 - The equilibrium constant K p for the following...Ch. 15 - At 1024°C, , the pressure of oxygen gas from the...Ch. 15 - 15.06 The equilibrium constant for the following...Ch. 15 - 15.107 Industrially, sodium metal is obtained by...Ch. 15 - Consider the equilibrium reaction described in...Ch. 15 - The K p for the reaction: SO 2 Cl 2 ( g ) ⇄ SO 2 (...Ch. 15 - The "boat" form and the “chair" form of...Ch. 15 - A quantity of 6.75 g of SO 2 Cl 2 was placed in a...Ch. 15 - 15.112 Industrial production of ammonia from...Ch. 15 - 15.113 The equilibrium constant for the formation...Ch. 15 - Consider the reaction: 2NO( g )+ O 2 ( g ) ⇄ 2N O...Ch. 15 - The formation of SO 3 from SO 2 and O 2 is an...Ch. 15 - At 25°C , the equilibrium partial pressures of N O...Ch. 15 - 15.117 The vapor pressure of mercury is 0.0020...Ch. 15 - 15.118 Both ' and are important biological ions....Ch. 15 - Photosynthesis can be represented by: 6C O 2 ( g...Ch. 15 - Consider the decomposition of ammonium chloride at...Ch. 15 - 15.121 Eggshells are composed mostly of calcium...Ch. 15 - In the gas phase, nitrogen dioxide is actually a...Ch. 15 - Consider the potential-energy diagrams for two...Ch. 15 - Iodine is sparingly soluble in water but much more...Ch. 15 - The dependence of the equilibrium constant of a...Ch. 15 - Lime ( CaO ) is used to prevent SO 2 from escaping...Ch. 15 - Lime is used to prevent from escaping from the...Ch. 15 - Lime ( CaO ) is used to prevent SO 2 from escaping...Ch. 15 - Lime ( CaO ) is used to prevent SO 2 from escaping...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Drawing of 3-fluro-2methylphenolarrow_forwardWhich compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forward
- HAND DRAWarrow_forwardPredict the major products of the following organic reaction: Some important notes: Δ CN ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. ONO reaction. Click and drag to start drawing a structure.arrow_forwardThe following product was made from diethyl ketone and what other reagent(s)? £ HO 10 2-pentyne 1-butyne and NaNH2 ☐ 1-propanol ☐ pyridine butanal ☐ pentanoatearrow_forward
- Which pair of reagents will form the given product? OH X + Y a. CH3 b. CH2CH3 ༧་་ C. CH3- CH2CH3 d.o6.(རི॰ e. CH3 OCH2CH3 -MgBr f. CH3-MgBr g. CH3CH2-MgBr -C-CH3 CH2CH3arrow_forwardQuestion 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forward
- Question 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forwardCould you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY