
(a)
Interpretation:
The statement “Aniline is an
(a)
Answer to Problem 5MCP
The given statement is true.
Explanation of Solution
Aniline is the simplest aromatic amine with the formula
(b)
Interpretation:
The statement “Aniline has a lower boiling point than benzoic acid” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(b)
Answer to Problem 5MCP
The given statement is true.
Explanation of Solution
Aniline is an amine and benzoic acid is
The ability of primary and secondary amines to form
(c)
Interpretation:
The statement “Aniline is planar molecule” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(c)
Answer to Problem 5MCP
The given statement is false because it is slightly pyramidalized molecule.
Explanation of Solution
Aniline is slightly pyramidalized molecule, with hybridization of the nitrogen between
Hence, the given statement is false.
(d)
Interpretation:
The statement “The molecular formula of aniline is
(d)
Answer to Problem 5MCP
The given statement is false because the molecular formula of aniline is
Explanation of Solution
Aniline is the simplest aromatic amine with the formula
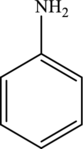
Hence, the statement “Aniline has a molecular formula of
(e)
Interpretation:
The statement “Aniline is a structural isomer of xylene” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(e)
Answer to Problem 5MCP
The given statement is false because both aniline and xylene have different molecular formulas.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of aniline is
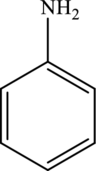
The molecular formula of xylene is
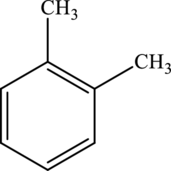
Both these are not structural isomers with each other.
Hence, the statement “aniline is structural isomer of xylene” is false because they have different molecular formulas.
(f)
Interpretation:
The statement “Aniline is more soluble than phenol” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(f)
Answer to Problem 5MCP
The given statement is false because both aniline and phenol are sparingly soluble in water.
Explanation of Solution
Aniline does not form hydrogen bonds with water to a very large extent due to the presence of large hydrophobic
The hydroxyl group of phenol is polar in nature, the oxygen is more electronegative than hydrogen, and hence it attracts shared electrons and acquires partial negative charge. The hydrogen of hydroxyl group in phenol carries partial negative charge. Phenol forms hydrogen bonding with water. However, the large part of phenol molecule is phenyl group is non-polar, thus making it sparingly soluble in water.
Hence, the given statement is false because both aniline and phenol are sparingly soluble in water.
(g)
Interpretation:
The statement “Aniline produces phenylammonium ion in water” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(g)
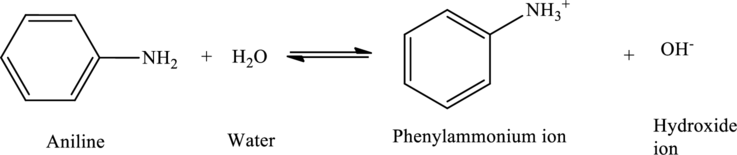
Answer to Problem 5MCP
The given statement is true.
Explanation of Solution
Aniline acts as bases, accepting
Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY(LL)-PKG
- CHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forward
- Draw the Fischer projection of D-fructose. Click and drag to start drawing a structure. Skip Part Check AP 14 tv SC F1 F2 80 F3 a F4 ! 2 # 3 CF F5 75 Ax MacBook Air 894 $ 5olo % Λ 6 > W F6 K F7 &arrow_forwardConsider this step in a radical reaction: Y What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ionization propagation initialization passivation none of the abovearrow_forward22.16 The following groups are ortho-para directors. (a) -C=CH₂ H (d) -Br (b) -NH2 (c) -OCHS Draw a contributing structure for the resonance-stabilized cation formed during elec- trophilic aromatic substitution that shows the role of each group in stabilizing the intermediate by further delocalizing its positive charge. 22.17 Predict the major product or products from treatment of each compound with Cl₁/FeCl₂- OH (b) NO2 CHO 22.18 How do you account for the fact that phenyl acetate is less reactive toward electro- philic aromatic substitution than anisole? Phenyl acetate Anisole CH (d)arrow_forward
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





