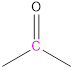
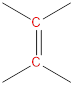
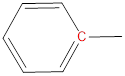
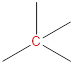
(a)
Interpretation: The number of signals and the chemical shift range of each signal to be predicted.
Concept Introduction:
The
Chemical shift range: The bench mark chemical shift region for the carbons of different electronic environments.
|
Carbon atoms of carbonyl groups are highly deshielded. |
|
|
|
(b)
Interpretation: The number of signals and the chemical shift range of each signal to be predicted.
Concept Introduction:
The
Chemical shift range: The bench mark chemical shift region for the carbons of different electronic environments.
|
Carbon atoms of carbonyl groups are highly deshielded. |
|
|
|
(c)
Interpretation: The number of signals and the chemical shift range of each signal to be predicted.
Concept Introduction:
The
Chemical shift range: The bench mark chemical shift region for the carbons of different electronic environments.
|
Carbon atoms of carbonyl groups are highly deshielded. |
|
|
|
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Organic Chemistry, 3e WileyPLUS Registration Card + Loose-leaf Print Companion
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Assign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forwarddescrive the energy levels of an atom and howan electron moces between themarrow_forwardRank each set of substituents using the Cahn-Ingold-Perlog sequence rules (priority) by numbering the highest priority substituent 1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY