
LL ORG CHEM
6th Edition
ISBN: 9781264840083
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 43P
Which compound in each pair is the stronger acid?
a. 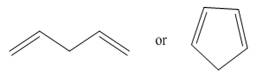 b.
b. 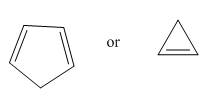
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting don't used Ai solution
Don't used hand raiting and don't used Ai solution
13.84. Chlorine atoms react with methane, forming HCI
and CH3. The rate constant for the reaction is
6.0 × 107 M¹ s¹ at 298 K. When the experiment
was run at three other temperatures, the following data
were collected:
T (K)
k (M-1 s-1)
303
6.5 × 107
308
7.0 × 107
313
7.5 x 107
a. Calculate the values of the activation energy and the
frequency factor for the reaction.
b. What is the value of the rate constant in the lower
stratosphere, where T = 218 K?
Chapter 15 Solutions
LL ORG CHEM
Ch. 15.2 - Prob. 1PCh. 15.2 - Problem 17.2 What orbitals are used to form the...Ch. 15.3 - Prob. 4PCh. 15.3 - Problem-17.5 What is the structure of propofol,...Ch. 15.6 - Prob. 7PCh. 15.8 - Prob. 8PCh. 15.8 - Prob. 11PCh. 15.8 - Prob. 12PCh. 15.8 - Problem 17.16 Rank the following compounds in...Ch. 15.8 - Problem 17.17 Draw the seven resonance structures...
Ch. 15 - 17.23 Name each compound and state how many lines...Ch. 15 - Prob. 21PCh. 15 - Prob. 22PCh. 15 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 15 - 17.28 Draw a structure corresponding to each...Ch. 15 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - 17.38
How many electrons does C contain?
How...Ch. 15 - Prob. 36PCh. 15 - 17.40 Explain the observed rate of reactivity of...Ch. 15 - 17.41 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 39PCh. 15 - 17.43 Draw additional resonance structures for...Ch. 15 - Prob. 41PCh. 15 - Prob. 42PCh. 15 - 17.46 Which compound in each pair is the stronger...Ch. 15 - 17.47 Treatment of indene with forms its...Ch. 15 - Prob. 45PCh. 15 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 15 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 15 - Prob. 48PCh. 15 - Prob. 49PCh. 15 - 17.53 How many signals does each compound...Ch. 15 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 15 - 17.55 Propose a structure consistent with each...Ch. 15 - 17.56 Propose a structure consistent with each...Ch. 15 - 17.57 Thymol (molecular formula ) is the major...Ch. 15 - 17.58 You have a sample of a compound of molecular...Ch. 15 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 15 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 15 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 15 - 17.62 Answer the following questions about...Ch. 15 - 17.63 Stanozolol is an anabolic steroid that...Ch. 15 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- My Organic Chemistry textbook says about the formation of cyclic hemiacetals, "Such intramolecular reactions to form five- and six-membered rings are faster than the corresponding intermolecular reactions. The two reacting functional groups, in this case OH and C=O, are held in close proximity, increasing the probability of reaction."According to the book, the formation of cyclic hemiacetals occurs in acidic conditions. So my question is whether the carbonyl group in this reaction reacts first with the end alcohol on the same molecule or with the ethylene glycol. And, given the explanation in the book, if it reacts first with ethylene glycol before its own end alcohol, why would it? I don't need to know the final answer. I need to know WHY it would not undergo an intermolecular reaction prior to reacting with the ethylene glycol if that is the case. Please do not use an AI answer.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardHighlight in red each acidic location on the organic molecule at left. Highlight in blue each basic location on the organic molecule at right. Note for advanced students: we mean acidic or basic in the Brønsted-Lowry sense only. Cl N شیخ x Garrow_forward
- Q4: Draw the mirror image of the following molecules. Are the molecules chiral? C/ F LL CI CH3 CI CH3 0 CI CH3 CI CH3 CH3arrow_forwardComplete combustion of a 0.6250 g sample of the unknown crystal with excess O2 produced 1.8546 g of CO2 and 0.5243 g of H2O. A separate analysis of a 0.8500 g sample of the blue crystal was found to produce 0.0465 g NH3. The molar mass of the substance was found to be about 310 g/mol. What is the molecular formula of the unknown crystal?arrow_forward4. C6H100 5 I peak 3 2 PPM Integration values: 1.79ppm (2), 4.43ppm (1.33) Ipeakarrow_forward
- Nonearrow_forward3. Consider the compounds below and determine if they are aromatic, antiaromatic, or non-aromatic. In case of aromatic or anti-aromatic, please indicate number of I electrons in the respective systems. (Hint: 1. Not all lone pair electrons were explicitly drawn and you should be able to tell that the bonding electrons and lone pair electrons should reside in which hybridized atomic orbital 2. You should consider ring strain- flexibility and steric repulsion that facilitates adoption of aromaticity or avoidance of anti- aromaticity) H H N N: NH2 N Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic TT electrons Me H Me Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic πT electrons H HH…arrow_forwardA chemistry graduate student is studying the rate of this reaction: 2 HI (g) →H2(g) +12(g) She fills a reaction vessel with HI and measures its concentration as the reaction proceeds: time (minutes) [IH] 0 0.800M 1.0 0.301 M 2.0 0.185 M 3.0 0.134M 4.0 0.105 M Use this data to answer the following questions. Write the rate law for this reaction. rate = 0 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY