
Organic Chemistry, 12e Study Guide/Student Solutions Manual
12th Edition
ISBN: 9781119077329
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 41P
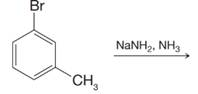
Predict the product of the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Pls help.
13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions
would be:
a. 1:1
b. 2:3
c. 3:2
d. 2:1
14) The pH of a 0.05 M solution of HCl(aq) at 25°C is
15) The pH of a 0.20 M solution of KOH at 25°C is
Pls help.
Chapter 15 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Ch. 15 - PRACTICE PROBLEM 15.1
Show how loss of a proton...Ch. 15 - Prob. 2PPCh. 15 - PRACTICE PROBLEM 15.3
Outline all steps in a...Ch. 15 - PRACTICE PROBLEM 15.4 Provide a mechanism that...Ch. 15 - Prob. 5PPCh. 15 - Prob. 6PPCh. 15 - Prob. 7PPCh. 15 - PRACTICE PROBLEM 15.8 Write resonance structures...Ch. 15 - PRACTICE PROBLEM 15.9
Provide a mechanism for the...Ch. 15 - PRACTICE PROBLEM 15.10 The trifluoromethyl group...
Ch. 15 - PRACTICE PROBLEM 15.11
Predict the major products...Ch. 15 - PRACTICE PROBLEM 15.12 Predict the major product...Ch. 15 - PRACTICE PROBLEM 15.13
Write mechanisms for the...Ch. 15 - Prob. 14PPCh. 15 - PRACTICE PROBLEM 15.15
Suppose you needed to...Ch. 15 - PRACTICE PROBLEM 15.16 1-Fluoro-2,4-dinitrobenzene...Ch. 15 - Prob. 17PPCh. 15 - PRACTICE PROBLEM 15.18
When...Ch. 15 - PRACTICE PROBLEM 15.19 Birch reduction of toluene...Ch. 15 - Prob. 20PCh. 15 - Prob. 21PCh. 15 - What monobromination product (or products) would...Ch. 15 - 15.23 Predict the major products of the following...Ch. 15 - Prob. 24PCh. 15 - 15.25 Starting with styrene, outline a synthesis...Ch. 15 - Prob. 26PCh. 15 - 15.27 Starting with aniline, outline a synthesis...Ch. 15 - Prob. 28PCh. 15 - Propose structures for compounds GI:Ch. 15 - 2,6-Dichlorophenol has been isolated from the...Ch. 15 - Prob. 31PCh. 15 - 15.32 Give structures (including stereochemistry...Ch. 15 - Provide a detailed mechanism for each of the...Ch. 15 - 15.34 Provide a detailed mechanism for the...Ch. 15 - Prob. 35PCh. 15 - Many polycyclic aromatic compounds have been...Ch. 15 - Prob. 37PCh. 15 - Prob. 38PCh. 15 - Prob. 39PCh. 15 - Prob. 40PCh. 15 - Predict the product of the following reaction.Ch. 15 - 15.42 When m-chlorotoluene is treated with sodium...Ch. 15 - Prob. 43PCh. 15 - Prob. 44PCh. 15 - Prob. 45PCh. 15 - Prob. 46PCh. 15 - 15.47 Provide structures for compounds A and B:
Ch. 15 - Prob. 48PCh. 15 - 15.49 Treating cyclohexene with acetyl chloride...Ch. 15 - 15.50 The tert-butyl group can be used as a...Ch. 15 - 15.51 When toluene is sulfonated (concentrated )...Ch. 15 - Prob. 52PCh. 15 - 2-Methylnaphthalene can be synthesized from...Ch. 15 - Prob. 54PCh. 15 - Prob. 55PCh. 15 - Prob. 56PCh. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Furan undergoes electrophilic aromatic...Ch. 15 - A C-D bond is harder to break than a C-H bond,...Ch. 15 - 15.61 Acetanilide was subjected to the following...Ch. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - When compound C, which is often used to model a...Ch. 15 - Open the molecular model file for benzyne and...Ch. 15 - The structure of thyroxine, a thyroid hormone that...Ch. 15 - Prob. 2LGPCh. 15 - 3. Deduce the structures of compounds E–L in the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
16. In a large metropolitan hospital, cells from newborn babies are collected and examined microscopically over...
Genetic Analysis: An Integrated Approach (3rd Edition)
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
Chemistry (7th Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
Figure 24-29 shows four arrangement? of charged particles, all the same distance from the origin. Rank the situ...
Fundamentals of Physics Extended
Johnny was vigorously exercising the only joints in the skull that are freely movable. What would you guess he ...
Anatomy & Physiology (6th Edition)
How did adding salt to solution B affect the travel time?
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help.arrow_forward16) A 2.0 L flask containing 2.0 x 10-3 mol H2(g), 3.0 x 10-3 mol Cl2(g), and 4.0 x 10-3 mol HCl(g) at equilibrium. This system is represented by the following chemical equation: H2 (g) + Cl2 (g) → 2HCl(g) Calculate the equilibrium constant for this reaction.arrow_forward7) The pH of a 0.05M solution of HCl(aq) at 25°C is a. 1.3 b. 2.3 c. 3.3 d. 12.7arrow_forward
- 11) The Ksp expression for copper (II) sulfate is: a. [Cu2+][SO4²¯] b. [Cu²+]² [SO4²]² c. [Cu²+]²[SO4²] d. [CuSO4] 12) Which of the following is true about a chemical system in equilibrium? a. All chemical reactions have stopped b. The concentration of reactants is equal to the concertation of products c. The forward and reverse reaction rates become equal d. The system will remain at equilibrium regardless of any external factorsarrow_forward21) Explain the difference between the rate of a reaction and the extent of a reaction. Why are both of these concepts important, if you are a chemical engineer that is trying to develop a process to produce a large volume of a specific type of chemical compound?arrow_forwardPls help.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY