
Concept explainers
(a)
Interpretation:
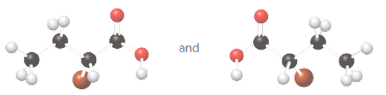
Whether the given pair of molecules are identical, or enantiomers should be determined.
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural arrangement (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
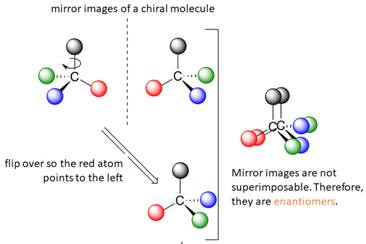
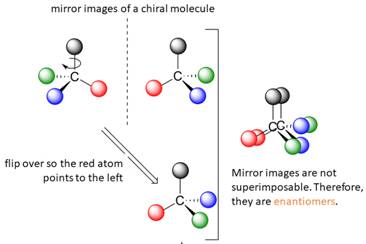
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
(b)
Interpretation:
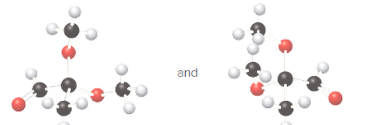
Whether the given pair of molecules are identical, or enantiomers should be determined.
Concept Introduction:
Identical molecules are the ones with the same chemical formula but no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural arrangement (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


