
ORGANIC CHEMISTRY W/CONNECT & ALEKS
6th Edition
ISBN: 9781264683888
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 35P
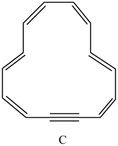
a. How many
b. How many
c. Explain why C is aromatic.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
a.How many π electrons does C contain?
b.How many π electrons are delocalized in the ring?
c.Explain why C is aromatic.
The purine heterocycle occurs commonly in the structure of DNA.
a. How is each N atom hybridized?
b. In what type of orbital does each lone pair on a N atom reside?
c. How many a electrons does purine contain?
d. Why is purine aromatic?
purine
INSTRUCTIONS: Choose the letter of the BEST answer for each item.
1. How many lone pairs are involved in sustaining the conjugation of pyridine?
A. One
B. Two
C. Three
D. Four
2. Benzopyrene, naphthalene and pyrene are members of these group of aromatic compounds:
A. Benzenoid aromatic compounds
B. Non-benzenoid aromatic compounds
C. Heterocyclic aromatic compounds
D. Heteronuclear compounds
3. What type of aromatic compound is pyridine?
A. Heterocyclic aromatic compound
B. Benzenoid aromatic compound
C. Non-benzenoid aromatic compound
D. Homonuclear cyclic compound
4. What property of aromatic rings prevent the involvement of the conjugated structure to addition reactions?
A. Radical stabilization
B. Resonance stability
C. Inductive effect
D. Aromatic effect
5. Which electrophilic aromatic substitution reaction is described when aniline is transformed into para-aminotolouene?
A. Nitration
B. Friedel Crafts alkylation
C. Oxidation
D. Halogenation
6. What type of relationship does…
Chapter 15 Solutions
ORGANIC CHEMISTRY W/CONNECT & ALEKS
Ch. 15.2 - Prob. 1PCh. 15.2 - Problem 17.2 What orbitals are used to form the...Ch. 15.3 - Prob. 4PCh. 15.3 - Problem-17.5 What is the structure of propofol,...Ch. 15.6 - Prob. 7PCh. 15.8 - Prob. 8PCh. 15.8 - Prob. 11PCh. 15.8 - Prob. 12PCh. 15.8 - Problem 17.16 Rank the following compounds in...Ch. 15.8 - Problem 17.17 Draw the seven resonance structures...
Ch. 15 - 17.23 Name each compound and state how many lines...Ch. 15 - Prob. 21PCh. 15 - Prob. 22PCh. 15 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 15 - 17.28 Draw a structure corresponding to each...Ch. 15 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - 17.38
How many electrons does C contain?
How...Ch. 15 - Prob. 36PCh. 15 - 17.40 Explain the observed rate of reactivity of...Ch. 15 - 17.41 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 39PCh. 15 - 17.43 Draw additional resonance structures for...Ch. 15 - Prob. 41PCh. 15 - Prob. 42PCh. 15 - 17.46 Which compound in each pair is the stronger...Ch. 15 - 17.47 Treatment of indene with forms its...Ch. 15 - Prob. 45PCh. 15 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 15 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 15 - Prob. 48PCh. 15 - Prob. 49PCh. 15 - 17.53 How many signals does each compound...Ch. 15 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 15 - 17.55 Propose a structure consistent with each...Ch. 15 - 17.56 Propose a structure consistent with each...Ch. 15 - 17.57 Thymol (molecular formula ) is the major...Ch. 15 - 17.58 You have a sample of a compound of molecular...Ch. 15 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 15 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 15 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 15 - 17.62 Answer the following questions about...Ch. 15 - 17.63 Stanozolol is an anabolic steroid that...Ch. 15 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why C is aromatic.arrow_forwardC. D. O: :O: The lone pair in compound C is Compound C is In compound D, not aromatic. aromatic. delocalized. not delocalized. one lone pair is delocalized. both lone pairs are not delocalized. both lone pairs are delocalized. Compoundarrow_forwardThe Diels–Alder reaction, a powerful reaction discussed in Chapter 14, occurs when a 1,3-diene such as A reacts with an alkene such as B to form the six-membered ring in C. a.Draw curved arrows to show how A and B react to form C. b.What bonds are broken and formed in this reaction? c.Would you expect this reaction to be endothermic or exothermic? d.Does entropy favor the reactants or products? e. Is the Diels–Alder reaction a substitution, elimination, or addition?arrow_forward
- Explain why A is a stable compound but B is not.arrow_forward41. Draw the structure of a hydrocarbon that has six carbon atoms and a. three vinylic hydrogens and two allylic hydrogens. b. three vinylic hydrogens and one allylic hydrogen. c. three vinylic hydrogens and no allylic hydrogens.arrow_forwardLabel each compound as aromatic, antiaromatic, or not aromatic. Assume all completely conjugated rings are planar. Å a. b. C. d.arrow_forward
- Which structure is aromatic and has six electrons In the conjugated system? Click on a letter A through D to answer. H A. C. D. В.arrow_forwardWhich of the following concepts explains why a tertiary carbocation is more stable than a primary carbocation? a. Hyperconjugation b. Resonance c. Electronegativity T d. he octet rulearrow_forwardWhich molecules below are aromatic? A. B. Structure D Structure B Structure A Structure C Structure E D. E.arrow_forward
- 13.arrow_forward6. Consider each compound below. Note the number of T electrons in the ring in each compound and indicate if it is aromatic or not aromatic (assume planarity for all molecules). B. TT electrons TT electrons TT electrons Aromatic? Aromatic? Aromatic?arrow_forwardfollowing Huckle rule, Which is not aromatic?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY