
(a)
Interpretation: For the given molecular formulae the structure should be predicted.
Concept Introduction:
1H NMR: A technique gives information to predict the Carbon and Hydrogen connectivity in the organic compounds.
HDI (Index of hydrogen deficiency): HDI values gives information on the level of unsaturation (means a double bond or a ring) on the structure of the compound.
(a)
Answer to Problem 32PP
Answer
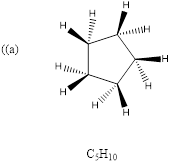
Explanation of Solution
To predict: the structure for the given molecular formula.
Calculate HDI Value for the given molecular formula and draw the structure.
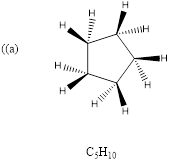
HDI = 1, compound has either 1 ring or one
Therefore, the compound predicted is the cyclopentane.
(b)
Interpretation: For the given molecular formulae the structure should be predicted.
Concept Introduction:
1H NMR: A technique gives information to predict the Carbon and Hydrogen connectivity in the organic compounds.
HDI (Index of hydrogen deficiency): HDI values gives information on the level of unsaturation (means a double bond or a ring) on the structure of the compound.
(b)
Answer to Problem 32PP
Answer
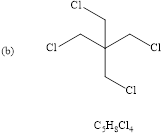
Explanation of Solution
The structure of given molecular formula.(
Calculate HDI value for the given molecular formula and draw the structure:

HDI = 0 , tells the compound is fully saturated (no double bond, triple bond or rings) and the compound is acyclic. Only one proton signal indicates all 4-
Therefore the predicted compound is 1, 3-dichoro-2, 2-dimethylchloropropane.
(c)
Interpretation: For the given molecular formulae the structure should be predicted.
Concept Introduction:
1H NMR: A technique gives information to predict the Carbon and Hydrogen connectivity in the organic compounds.
HDI (Index of hydrogen deficiency): HDI values gives information on the level of unsaturation (means a double bond or a ring) on the structure of the compound.
(c)
Answer to Problem 32PP
Answer
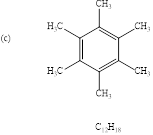
Explanation of Solution
To find: the structure of given molecular formula.
Calculate HDI value for the given molecular formula and draw the structure:
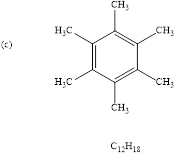
HDI = 4, shows the compound is
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Assign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forwarddescrive the energy levels of an atom and howan electron moces between themarrow_forwardRank each set of substituents using the Cahn-Ingold-Perlog sequence rules (priority) by numbering the highest priority substituent 1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





