
Concept explainers
PRACTICE PROBLEM 15.1
Show how loss of a proton can be represented using each of the three resonance structures for the arenium ion and show how each representation leads to the formation of a benzene ring with three alternating double bonds (i.e., six fully delocalized
Interpretation:
The loss of protons from three resonating structures of the areniumion, are to be shown.
Concept Introduction:
Arrows present in the mechanism of the reaction indicate the electron transfer, starting from negatively charged species and ending at the positively charged species.
Electrophiles attract negatively charged species and nucleophiles attract positively charged species.
Answer to Problem 1PP
Solution: The loss of protons from the three resonating structures of the arenium ion are shown as follows:
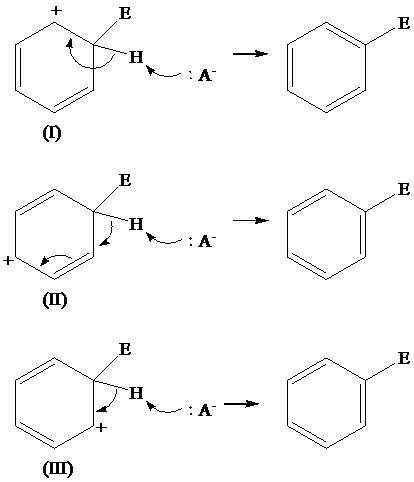
Explanation of Solution
The resonating structures of the arenium ion, formed in the electrophilic substitution reaction of benzene, are shown below:

The removal of proton occurs from the carbon atom where the electrophile is attached and the bond pairs of electrons of
The removal of proton from (I) resonating structure is shown as follows:
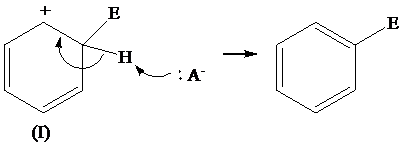
The removal of proton from (II) resonating structure is shown as follows:
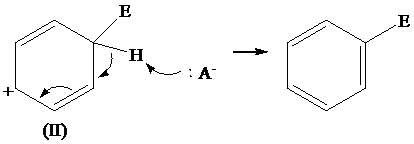
The removal of proton from (III) resonating structure is shown as follows:
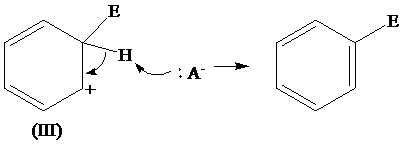
The removal of protons from the arenium ion leads to the transfer of bond pairs to the benzene ring and the delocalization of these electrons on the ring occurs, to form the final product.
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
Additional Science Textbook Solutions
Fundamentals of Anatomy & Physiology (11th Edition)
Organic Chemistry (8th Edition)
Principles of Anatomy and Physiology
Chemistry: The Central Science (14th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Living By Chemistry: First Edition Textbook
- At a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forward
- How to name hydrocarbonsarrow_forwardPlease do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.arrow_forwardWhen two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)arrow_forward
- Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

