
Concept explainers
16-6 Answer true or false.
- te/7-Butylamine is a 3°
amine .
(a)
Interpretation:
To analyse whether the given statement: tert-Butylamine is
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 1P
The statement is false.
Explanation of Solution
The structure of a tert-butylamine is given below:
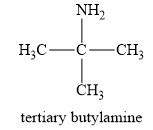
In a tert-butylamine, the carbon atom is bonded to three methyl groups, while nitrogen is attached to only one carbon group. Therefore, the tert-butyl amine is a primary amine. Therefore, this statement is false.
(b)
Interpretation:
To analyse whether the given statement about the aromatic amine is true or not.
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 1P
The statement is true.
Explanation of Solution
If in an amine, the nitrogen is directly bonded to one or more aryl groups or aromatic rings, and then the amine is known as an aromatic amine.
For example, aniline is an aromatic amine, as it is attached to one aromatic ring benzene. The structure of aniline is given below:
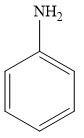
Therefore, the given statement is true.
(c)
Interpretation:
To analyse whether the given statement: In a heterocyclic amine, the main nitrogen is the part of the ringis true or not.
Concept Introduction:
Amines are the derivatives of ammonia, wherein one or more than one hydrogen atoms are substituted by an alkyl or aryl group.
The amines are categorized as primary, secondary or tertiary based on the number of carbon atoms that are bonded directly to the nitrogen atom. Primary amine has only one carbon atom bonded to the nitrogen atom. Similarly, secondary amines have two carbon groups bonded to the nitrogen, and tertiary amines nave three carbon groups bonded to the nitrogen.
Answer to Problem 1P
The statement is true.
Explanation of Solution
In a heterocyclic amine, the carbon group of the ring structure is replaced by the nitrogen atom. For example, in pyridine, the nitrogen atom replaces one —CH group of the benzene ring.
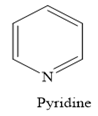
Thus, the heterocyclic amine is an amine in which nitrogen is one of the atoms of a ring. Therefore, this statement is true.
(d)
Interpretation:
To analyze whether the given statement: The NH4+ and CH4have the same number of valence electrons and both have tetrahedral geometry as per the VSEPR model is true or not.
Concept Introduction:
The VSEPR (Valence-shell electron pair repulsion) model predicts the shape of the molecules or ions by identifying the position of atoms connected to the central atom.
Answer to Problem 1P
The statement is true.
Explanation of Solution
The valence shell electron pair repulsion (VSEPR) theory determines the shapes and the geometry of a molecule. In a CH4 molecule, one carbon atom is bonded to four hydrogen atoms with a single bond. In NH4+, one nitrogen atom is singly bonded to four hydrogen atoms.
According to the VSEPR model, each bond represents a pair of electrons. Since both compounds have four bonds around the central atom, they contain eight valence electrons. Also, according to the VSEPR model, these four bonding regions are arranged in a tetrahedral manner so that they are as far away from one another as possible, giving the molecule a tetrahedral geometry. Therefore, this statement is true.
(e)
Interpretation:
To analyze whether the given statement: There are four constitutional isomers with the molecular formulaC3H9N, is true or not.
Concept Introduction:
Constitutional isomers are those compounds which have same molecular formula, but they differ in the arrangement of atoms in the molecules.
Answer to Problem 1P
The statement is true.
Explanation of Solution
The molecular formula is given as C3H9N. Therefore, the possible constitutional isomers for this molecular formula are given below:
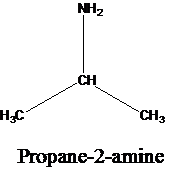
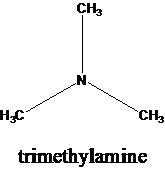
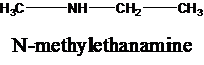
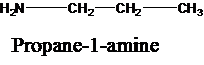
Thus, there are four constitutional isomers possible with the molecular formula C3H9N. Therefore, this statement is true.
Want to see more full solutions like this?
Chapter 15 Solutions
Introduction To General, Organic, And Biochemistry
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

