
(a)
Interpretation:
The statement “
(a)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
Alcohols have strong intermolecular hydrogen bonding. The
On the other hand, the polarity of the amine nitrogen is less than the oxygen in alcohol. The nitrogen amine is less electronegative than oxygen in the alcohol. Therefore, the dipole on
(b)
Interpretation:
The statement “
(b)
Answer to Problem 1MCP
The given statement is false because of the presence of strong intermolecular hydrogen bonding in octanoic acid, it has higher boiling point than
Explanation of Solution
Carboxylic acids have strong intermolecular hydrogen bonding with each other and strong dipole-dipole attractions. The presence of polar carboxyl group and intermolecular hydrogen bonding makes these acids have higher boiling points. The presence of dimers increases the strength of the van der Waals dispersion forces, which makes them have high boiling points.
The ability of primary and secondary amines to form
Hence, the statement “
(c)
Interpretation:
The statement “
(c)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
Amine with five or fewer carbon atoms is soluble in water.
(d)
Interpretation:
The statement “
(d)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
In
(e)
Interpretation:
The line formula of
(e)
Answer to Problem 1MCP
The given line formula of
Explanation of Solution
The given line formula is,

The above line formula represents
(f)
Interpretation:
The statement “
(f)
Answer to Problem 1MCP
The given statement is false because
Explanation of Solution
(g)
Interpretation:
The statement “
(g)
Answer to Problem 1MCP
The given statement is false because it secondary amine.
Explanation of Solution
The structure of

From the structure of
(h)
Interpretation:
The statement “
(h)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
Reduction of amides in the presence of
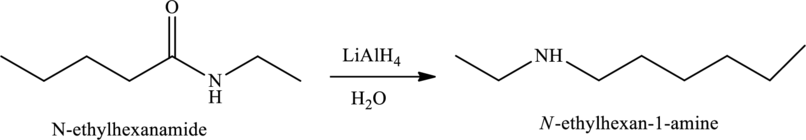
(i)
Interpretation:
The statement “
(i)
Answer to Problem 1MCP
The given statement is false because they have different structural and molecular formulas.
Explanation of Solution
The structure of
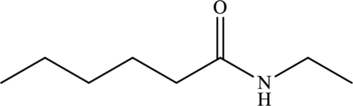
The structure of

(j)
Interpretation:
The statement “The reaction of
(j)
Answer to Problem 1MCP
The given statement is false because the neutralization reaction of the
Explanation of Solution
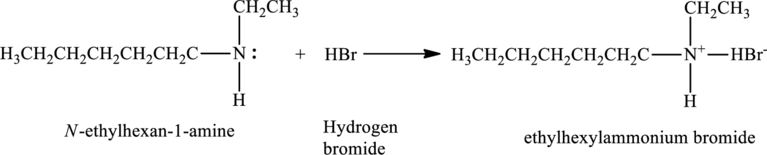
Hence the given statement is false because the neutralization reaction of
(k)
Interpretation:
The statement “The alkyl ammonium salt would be more water soluble than the amine” has to be predicted as true or false. If the statement is false, the reason for the false statement has to be described.
(k)
Answer to Problem 1MCP
The given statement is true.
Explanation of Solution
By adding a strong acid to a water-insoluble amine, a water-soluble alkylammonium salt can be formed. The salt could be converted back to an amine by reaction with strong base.
Alkyl ammonium salts can neutralize hydroxide ions. Water is formed and the protonated amine cation is converted into an amine. The nitrogen atom of an ammonium salt has a positive charge, alkyl ammonium salts are more water-soluble than amines.
(l)
Interpretation:
The statement “
(l)
Answer to Problem 1MCP
The given statement is false because it is not a cyclic compound.
Explanation of Solution
The structure of

Want to see more full solutions like this?
Chapter 15 Solutions
GENERAL, ORGANIC, BIOCHEM (LL W/ ACCESS)
- Nonearrow_forwardDraw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forward
- Nonearrow_forwardChoose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forward
- Part II. count the expected number of signals in the 1H-NMR spectrum of these compounds HO 0 одев * Cl -cl "D"arrow_forwardPart I. Create a splitting tree diagram to predict the multiplet pattern of proton Hb in the compound below: 3 (Assume that "Jab >>> ³JbC) Ha Hb He он Ha NH2 Ha HCarrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





