
Concept explainers
(a)
Interpretation:
A concept map is to be drawn and the grams of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 19E
The concept map is shown below.
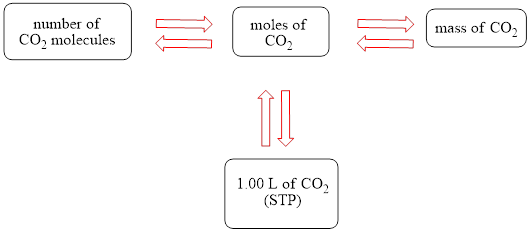
The grams of
Explanation of Solution
When

Figure 1
The volume occupied by
Therefore, the number of moles which occupy
The molar mass of
Therefore, the mass of
The formula to calculate the mass of
Substitute the mass of
Therefore, the grams of
The grams of
(b)
Interpretation:
A concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 19E
The concept map is shown below.

The molecules of
Explanation of Solution
When

Figure 1
The volume occupied by
Therefore, the number of moles which occupy
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(c)
Interpretation:
A concept map is to be drawn and the molar concentration of the carbonic acid solution when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 19E
The concept map is shown below.

The molar concentration of the carbonic acid solution is
Explanation of Solution
When

Figure 1
The volume occupied by
Therefore, the number of moles which occupy
The number of moles in
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of carbonic acid solution is
Want to see more full solutions like this?
Chapter 15 Solutions
EBK INTRODUCTORY CHEMISTRY
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





