
(a)
Interpretation:
The average rate of decomposition of
Concept Introduction:
(a)
Answer to Problem 17E
The average rate of decomposition of
Explanation of Solution
The reaction is given as follows:
The given data is shown below:
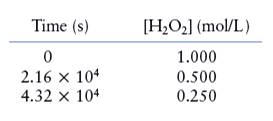
The rate of the reaction is given as follows:
The average rate of decomposition of
Therefore, the average rate of decomposition of
Similarly, the average rate of production of
Therefore, the average rate of production of
(b)
Interpretation:
The average rate of decomposition of
Concept Introduction:
Rate of reaction represents the change of concentration of a reactant or a product with respect to time. It can be expressed either by the reduce amount of reactant in per unit time or increase the amount of product in per unit time.
(b)
Answer to Problem 17E
The average rate of decomposition of
Explanation of Solution
The reaction is given as follows:
The given data is shown below:
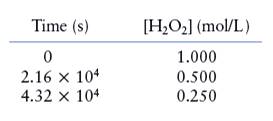
The rate of the reaction is given as follows:
The average rate of decomposition of
Therefore, the average rate of decomposition of
Similarly, the average rate of production of
Therefore, the average rate of production of
Want to see more full solutions like this?
Chapter 15 Solutions
EBK WEBASSIGN FOR ZUMDAHL'S CHEMICAL PR
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





