
Interpretation:
Fatty acids are carbon chains with a methyl group at one end and carboxyl 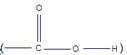 group at the other end.
group at the other end.
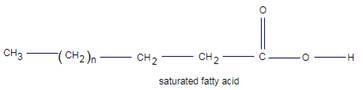
In the fatty acid middle portion may be saturated or unsaturated.
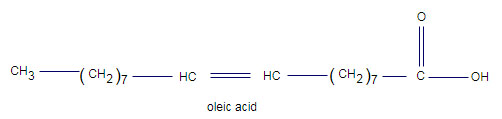
Monounsaturated fatty acids have one carbon positions. This monounsaturated fatty acid has two form: cis and trans. Trans isomers is produced during industrial processing (hydrogenation) of unsaturated oils Cis and Trans form of monounsaturated fatty acid show different physical and chemical property.
Concept introduction:
The presence of double bond causes restriction in the mobility of acyl chain. The cis configuration gives a knick in the molecule in the molecules. The cis fatty acid is less stable than trans fatty acid. The cis fatty acid has lower meeting point than the Trans.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- Don't used hand raitingarrow_forwardIf a high molecular weight linear polyethylene is chlorinated by inducing the substitution of chlorine atoms by hydrogen, if 5% of all hydrogen atoms are replaced, what approximate percentage of chlorine by weight would the product have?arrow_forwardO Macmillan Learning Chemistry: Fundamentals and Principles Davidson presented by Macmillan Learning Poly(ethylene terephthalate), known as PET or industrially as Dacron, is a polyester synthesized through a condensation reaction between two bifunctional monomers. The monomers, ethylene glycol and terepthalic acid, are given. Add bonds and remove atoms as necessary to show the structure of a two repeat unit portion of a longer polymer chain of PET. You may need to zoom out to see the complete structure of all four monomer units. Select Draw / || | C H 0 3 © Templates More ° ° ° || C CC - OH HO OH HOC - C Erase CC OH HO C C 〃 C H₂ Q2Qarrow_forward
- Q1 - What type(s) of bonding would be expected for each of the following materials: solid xenon, calcium fluoride (CaF2), bronze, cadmium telluride (CdTe), rubber, and tungsten? Material solid xenon CaF2 bronze CdTe rubber tungsten Type(s) of bonding Q2- If the atomic radius of lead is 0.175 nm, calculate the volume of its unit cell in cubic meters.arrow_forwardDetermine the atomic packing factor of quartz, knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are 0.038 and 0.117 nm.arrow_forwardUse the following data for an unknown gas at 300 K to determine the molecular mass of the gas.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





