
Organic Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (9th Edition) (New in Organic Chemistry)
9th Edition
ISBN: 9780321971128
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.28SP
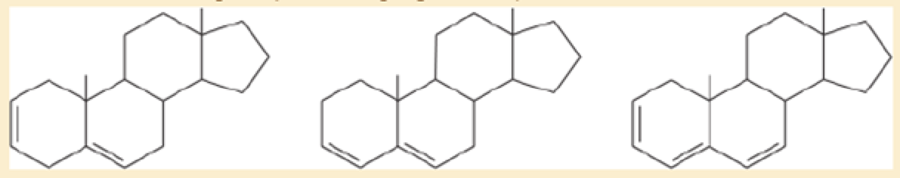
A solution was prepared using 0.0010 g of an unknown steroid (of molecular weight around 255) in 100 mL of ethanol. Some of this solution was placed in a 1-cm cell, and the UV spectrum was measured. This solution was found to have λmax=235nm, with A=0.74.
- a. Compute the value of the molar absorptivity at 235 nm.
- b. Which of the following compounds might give this spectrum?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 15 Solutions
Organic Chemistry Plus Mastering Chemistry with Pearson eText -- Access Card Package (9th Edition) (New in Organic Chemistry)
Ch. 15.2 - Prob. 15.1PCh. 15.2 - Prob. 15.2PCh. 15.2 - Prob. 15.3PCh. 15.4 - Prob. 15.4PCh. 15.4 - Prob. 15.5PCh. 15.5 - Treatment of an alkyl halide with AgNO3 in alcohol...Ch. 15.5 - Propose a mechanism for each reaction, showing...Ch. 15.6 - When Br2 is added to buta-1,3-diene at 15 C, the...Ch. 15.7 - Prob. 15.9PCh. 15.7 - When N-bromosuccinimide is added to hex-1-ene in...
Ch. 15.7 - Prob. 15.11PCh. 15.9 - Addition of 1-bromobut-2-ene to magnesium metal in...Ch. 15.10 - Show how you might synthesize the following...Ch. 15.11 - Predict the products of the following proposed...Ch. 15.11 - Prob. 15.15PCh. 15.11A - Prob. 15.16PCh. 15.11B - Prob. 15.17PCh. 15.11B - Predict the products of the following Diels-Alder...Ch. 15.12C - Prob. 15.19PCh. 15.12C - Prob. 15.20PCh. 15.13C - Prob. 15.21PCh. 15.13D - Using the examples in Table15-2 to guide you,...Ch. 15.14 - Phenolphthalein is an acid-base indicator that is...Ch. 15 - Prob. 15.24SPCh. 15 - Prob. 15.25SPCh. 15 - Show how the reaction of an allylic halide with a...Ch. 15 - Prob. 15.27SPCh. 15 - A solution was prepared using 0.0010 g of an...Ch. 15 - Prob. 15.29SPCh. 15 - Prob. 15.30SPCh. 15 - Prob. 15.31SPCh. 15 - Prob. 15.32SPCh. 15 - Prob. 15.33SPCh. 15 - Give the structures of the products represented by...Ch. 15 - Furan and malemide undergo a Diels-Alder reaction...Ch. 15 - Prob. 15.36SPCh. 15 - Prob. 15.37SPCh. 15 - Prob. 15.38SPCh. 15 - Prob. 15.39SPCh. 15 - Determine whether each structure is likely to be...Ch. 15 - An important variation of the Diels-Alder reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY