
Concept explainers
(a)
Interpretation:
The object, a circular clock face is asymmetric or not has to be given.
Concept Introduction:
Symmetric:
When a molecule is rotated by an axis of symmetry, the original and the rotated species will be indistinguishable from one another are said to be symmetric. In simple terms, when a molecule is divided into two parts, if the two parts are equal means they have symmetry.
Example:
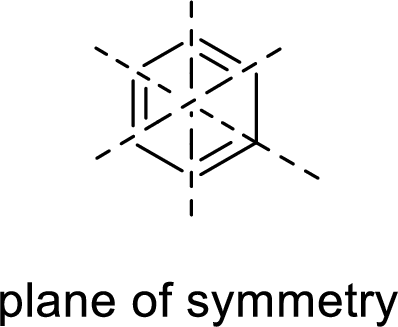
Asymmetric:
The molecules which has no plane of symmetry or center of symmetry are said to be asymmetric. It depends on the presence of asymmetric atom in the molecule. When a molecule is divided into two parts, if the two parts are unequal means they are asymmetric.
Example:
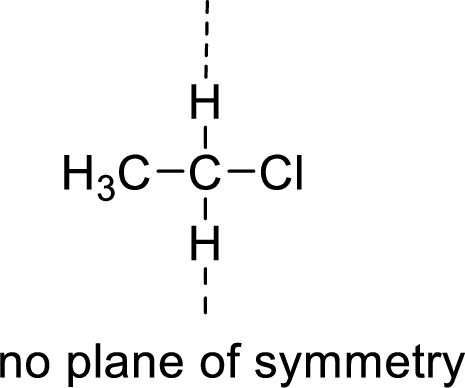
(b)
Interpretation:
The object, a football is asymmetric or not has to be given.
Concept Introduction:
Symmetric:
When a molecule is rotated by an axis of symmetry, the original and the rotated species will be indistinguishable from one another are said to be symmetric. In simple terms, when a molecule is divided into two parts, if the two parts are equal means they have symmetry.
Example:
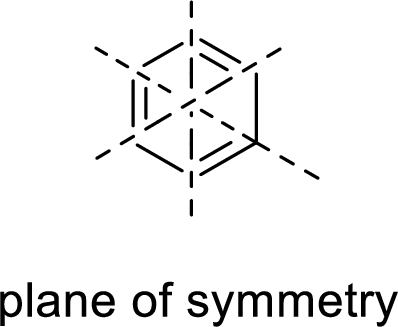
Asymmetric:
The molecules which has no plane of symmetry or center of symmetry are said to be asymmetric. It depends on the presence of asymmetric atom in the molecule. When a molecule is divided into two parts, if the two parts are unequal means they are asymmetric.
Example:
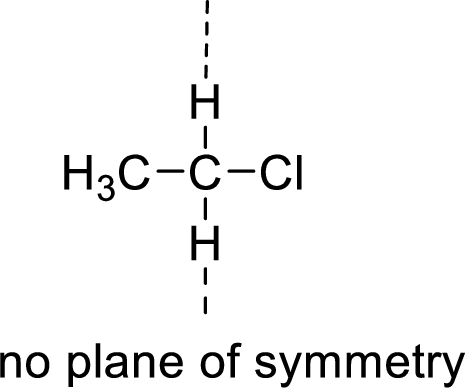
(c)
Interpretation:
The object, a dime is asymmetric or not has to be given.
Concept Introduction:
Symmetric:
When a molecule is rotated by an axis of symmetry, the original and the rotated species will be indistinguishable from one another are said to be symmetric. In simple terms, when a molecule is divided into two parts, if the two parts are equal means they have symmetry.
Example:
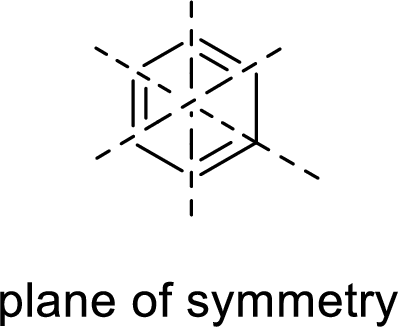
Asymmetric:
The molecules which has no plane of symmetry or center of symmetry are said to be asymmetric. It depends on the presence of asymmetric atom in the molecule. When a molecule is divided into two parts, if the two parts are unequal means they are asymmetric.
Example:
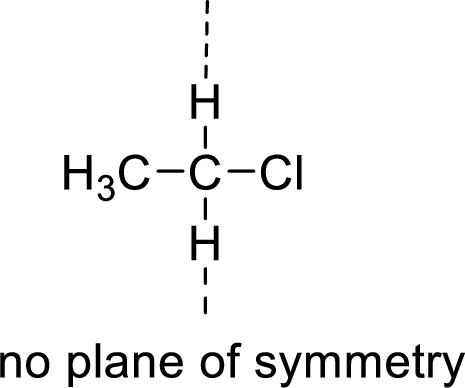
(d)
Interpretation:
The object, a brick is asymmetric or not has to be given.
Concept Introduction:
Symmetric:
When a molecule is rotated by an axis of symmetry, the original and the rotated species will be indistinguishable from one another are said to be symmetric. In simple terms, when a molecule is divided into two parts, if the two parts are equal means they have symmetry.
Example:
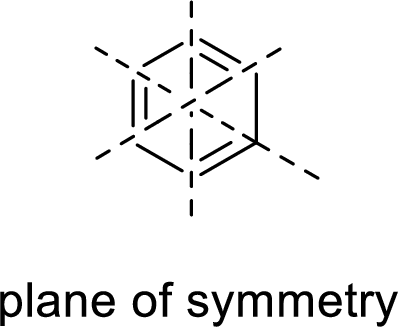
Asymmetric:
The molecules which has no plane of symmetry or center of symmetry are said to be asymmetric. It depends on the presence of asymmetric atom in the molecule. When a molecule is divided into two parts, if the two parts are unequal means they are asymmetric.
Example:
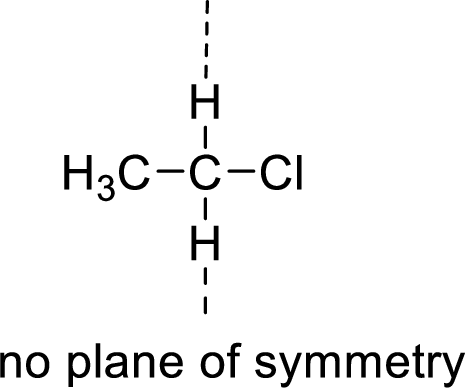
(e)
Interpretation:
The object, a hammer is asymmetric or not has to be given.
Concept Introduction:
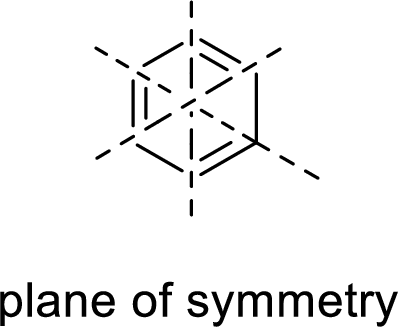
Symmetric:
When a molecule is rotated by an axis of symmetry, the original and the rotated species will be indistinguishable from one another are said to be symmetric. In simple terms, when a molecule is divided into two parts, if the two parts are equal means they have symmetry.
Example:
Asymmetric:
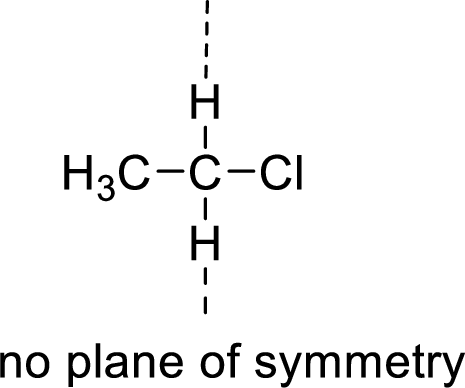
The molecules which has no plane of symmetry or center of symmetry are said to be asymmetric. It depends on the presence of asymmetric atom in the molecule. When a molecule is divided into two parts, if the two parts are unequal means they are asymmetric.
Example:
(f)
Interpretation:
The object, a spring is asymmetric or not has to be given.
Concept Introduction:
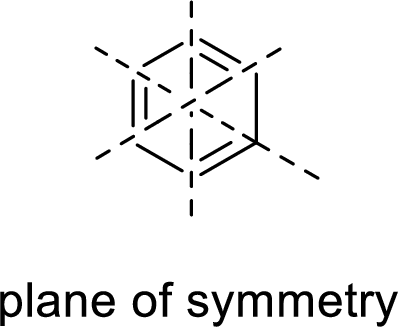
Symmetric:
When a molecule is rotated by an axis of symmetry, the original and the rotated species will be indistinguishable from one another are said to be symmetric. In simple terms, when a molecule is divided into two parts, if the two parts are equal means they have symmetry.
Example:
Asymmetric:
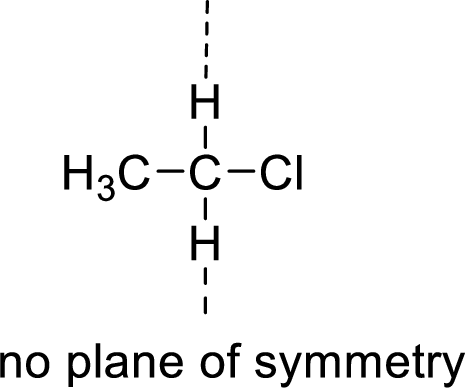
The molecules which has no plane of symmetry or center of symmetry are said to be asymmetric. It depends on the presence of asymmetric atom in the molecule. When a molecule is divided into two parts, if the two parts are unequal means they are asymmetric.
Example:
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Loose Leaf for Chemistry: The Molecular Nature of Matter and Change
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





