
College Physics
1st Edition
ISBN: 9781938168000
Author: Paul Peter Urone, Roger Hinrichs
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 14PE
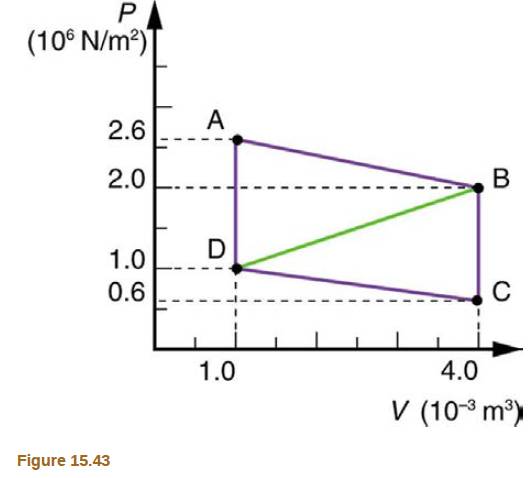
Calculate the net work output of a
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Identical rays of light enter three transparent blocks composed of
different materials. Light slows down upon entering the blocks.
For single-slit diffraction, calculate the first three values of (the total phase difference between rays from each
edge of the slit) that produce subsidiary maxima by a) using the phasor model, b) setting dr = 0, where I is
given by,
I
=
Io (sin (10) ².
2
A capacitor with a capacitance of C = 5.95×10−5 F is charged by connecting it to a 12.5 −V battery. The capacitor is then disconnected from the battery and connected across an inductor with an inductance of L = 1.55 H .
(D)What is the charge on the capacitor 0.0235 s after the connection to the inductor is made? Interpret the sign of your answer. (e) At the time given in part (d), what is the current in the inductor? Interpret the sign of your answer. (f) Atthe time given in part (d), how much electrical energy is stored in the capacitor and how much is stored in the inductor?
Chapter 15 Solutions
College Physics
Ch. 15 - Describe the photo of the tea kettle at the...Ch. 15 - The first law of thermodynamics and the...Ch. 15 - Heat transfer Q and work done W are always energy...Ch. 15 - How do heat transfer and internal energy differ?...Ch. 15 - If you run down some stairs and stop, what happens...Ch. 15 - Give an explanation of how food energy (calories)...Ch. 15 - Identify the type of energy transferred to your...Ch. 15 - A great deal of effort time, and money has been...Ch. 15 - One method of converting heat transfer to doing...Ch. 15 - Would the previous question make any sense for an...
Ch. 15 - We ordinarily say that U=0 for an isothermal...Ch. 15 - The temperature of a rapidly expanding gas...Ch. 15 - Which cyclical process represented by the two...Ch. 15 - A real process may be nearly adiabatic if it...Ch. 15 - It is unlikely that a process can be isothermal...Ch. 15 - Imagine you are driving a car up Pike’s Peak in...Ch. 15 - Is a temperature difference necessary to operate a...Ch. 15 - Definitions of efficiency vary depending on how...Ch. 15 - Whyother than the fact that the second law of...Ch. 15 - Think about the drinking bird at the beginning of...Ch. 15 - Can improved engineering and materials be employed...Ch. 15 - Does the second law of thermodynamics alter the...Ch. 15 - Explain why heat pumps do not work as well in very...Ch. 15 - In some Northern European nations, homes are being...Ch. 15 - Why do refrigerators, air conditioners, and heat...Ch. 15 - Grocery store managers contend that there is less...Ch. 15 - Can you cool a kitchen by leaving the refrigerator...Ch. 15 - A woman shuts her summer cottage up in September...Ch. 15 - Consider a system with a certain energy content,...Ch. 15 - Does a gas become more orderly when it liquefies?...Ch. 15 - Explain how water’s entropy can decrease when it...Ch. 15 - Is a uniform-temperature gas more or less orderly...Ch. 15 - Give an example of a spontaneous process in which...Ch. 15 - What is the change in entropy in an adiabatic...Ch. 15 - Does the entropy at a star increase or decrease as...Ch. 15 - Explain why a building made of bricks has smaller...Ch. 15 - Explain why a building made of bricks has smaller...Ch. 15 - What is the change in internal energy of a car if...Ch. 15 - How much heat transfer occurs from a system, if...Ch. 15 - A system does 1.80108J of work while 7.50108J of...Ch. 15 - What is the change in internal energy of a system...Ch. 15 - Suppose a woman does 500 J of work and 9500 J of...Ch. 15 - (a) How much food energy will a man metabolize in...Ch. 15 - (a) What is the average metabolic rate in watts of...Ch. 15 - (a) How long will the energy in a 1470kJ (350kcal)...Ch. 15 - (a) A woman climbing the Washington Monument...Ch. 15 - A car tire contains 0.0380m3 S of air at a...Ch. 15 - A heliumfilled toy balloon has a gauge pressure of...Ch. 15 - Steam to drive an old—fashioned steam locomotive...Ch. 15 - A hand—driven tire pump has a piston with a 2.50cm...Ch. 15 - Calculate the net work output of a heat engine...Ch. 15 - What is the net work output of a heat engine that...Ch. 15 - Unreasonable Results What is wrong with the claim...Ch. 15 - (a) A cyclical heat engine, operating between...Ch. 15 - Construct Your Own Problem Consider a car's...Ch. 15 - Construct Your Own Problem Consider a car trip...Ch. 15 - A certain heat engine does 10.0 kJ of work and...Ch. 15 - With 2.56106J of heat transfer into this engine, a...Ch. 15 - (a) What is the work output of a cyclical heat...Ch. 15 - (a) What is the eficiency of a cyclical heat...Ch. 15 - The engine of a large Ship does 2.00108J of work...Ch. 15 - (a) How much heat transfer occurs to the...Ch. 15 - Assume that the turbines at a coal—powered power...Ch. 15 - This problem compares the energy output and heat...Ch. 15 - A certain gasoline engine has an efficiency of...Ch. 15 - A gascooled nuclear reactor operates between hot...Ch. 15 - (a) What is the hot reservoir temperature of a...Ch. 15 - Steam locomotives have an efficiency of 17.0% and...Ch. 15 - Practical steam engines utilize 450C steam, which...Ch. 15 - A coalfired electrical power station has an...Ch. 15 - Would you be willing to financially back an...Ch. 15 - Unreasonable Results (a) Suppose you want to...Ch. 15 - Unreasonable Results Calculate the cold reservoir...Ch. 15 - What is the coefficient of performance of an ideal...Ch. 15 - Suppose you have an ideal refrigerator that cools...Ch. 15 - What is the best coefficient of performance...Ch. 15 - In a very mild winter climate, a heat pump has...Ch. 15 - (a) What is the best coefficient of performance...Ch. 15 - (a) What is the best coefficient of performance...Ch. 15 - Suppose you want to operate an ideal refrigerator...Ch. 15 - An ideal heat pump is being considered for use in...Ch. 15 - A 4ton air conditioner removes 5.60107J (48,000...Ch. 15 - Show that the coefficients of performance of...Ch. 15 - (a) On a winter day, a certain house loses...Ch. 15 - On a hot summer day, 4.00106J of heat transfer...Ch. 15 - A hot rock ejected from a volcano's lava fountain...Ch. 15 - When 1.60105J of heat transfer occurs into a meat...Ch. 15 - The Sun radiates energy at the rate of 3.801026W...Ch. 15 - (a) In reaching equilibrium, how much heat...Ch. 15 - What is the decrease in entropy of 25.0 g of water...Ch. 15 - Find the increase in entropy of 1.00 kg of liquid...Ch. 15 - A large electrical power station generates 1000 MW...Ch. 15 - (a) How much heat transfer occurs from 20.0 kg of...Ch. 15 - Using Table 15.4, verify the contention that if...Ch. 15 - What percent of the time will you get something in...Ch. 15 - (a) If tossing 100 coins, how many ways...Ch. 15 - (a) What is the change in entropy if you start...Ch. 15 - (a) What is the change in entropy if you start...Ch. 15 - (a) If you toss 10 coins, what percent of the time...Ch. 15 - (a) Construct a table showing the macro states and...Ch. 15 - In an air conditioner, 12.65 MJ of heat transfer...
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
Correct any answers that have the incorrect number of significant figures. a. (7856944)456=3152 b. (89105234810...
Introductory Chemistry (6th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
41. A 0.300 kg oscillator has a speed of 95.4cm/s when its displacement is 3.00cm and 71.4 cm/s when its displ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautome...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Close-up view etermine; The volume of the object given that the initial level of water in the measuring cylinder 23cm3. The density of the object. simple cell made by dipping copper and zinc plates into dilute sulfuric acid solution. A bull onnected across the plates using a wire. State what constitute current flow through the wire The bulb connected across is observed to light for some time and then goes out. State t possible asons for this observation. State two ways in which the processes named in question (b) above can be minimized t the bulb light for a longer period. ead is rated 80Ah. Determine the current that can be drawn continuouslyarrow_forwardAnswers with -1.828, -1.31 or 939.3 are not correct.arrow_forwardThree slits, each separated from its neighbor by d = 0.06 mm, are illuminated by a coherent light source of wavelength 550 nm. The slits are extremely narrow. A screen is located L = 2.5 m from the slits. The intensity on the centerline is 0.05 W. Consider a location on the screen x = 1.72 cm from the centerline. a) Draw the phasors, according to the phasor model for the addition of harmonic waves, appropriate for this location. b) From the phasor diagram, calculate the intensity of light at this location.arrow_forward
- A Jamin interferometer is a device for measuring or for comparing the indices of refraction of gases. A beam of monochromatic light is split into two parts, each of which is directed along the axis of a separate cylindrical tube before being recombined into a single beam that is viewed through a telescope. Suppose we are given the following, • Length of each tube is L = 0.4 m. • λ= 598 nm. Both tubes are initially evacuated, and constructive interference is observed in the center of the field of view. As air is slowly let into one of the tubes, the central field of view changes dark and back to bright a total of 198 times. (a) What is the index of refraction for air? (b) If the fringes can be counted to ±0.25 fringe, where one fringe is equivalent to one complete cycle of intensity variation at the center of the field of view, to what accuracy can the index of refraction of air be determined by this experiment?arrow_forward1. An arrangement of three charges is shown below where q₁ = 1.6 × 10-19 C, q2 = -1.6×10-19 C, and q3 3.2 x 10-19 C. 2 cm Y 93 92 91 X 3 cm (a) Calculate the magnitude and direction of the net force on q₁. (b) Sketch the direction of the forces on qiarrow_forward(Figure 1)In each case let w be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w Find the direction of the force exerted on the strut by the pivot in the arrangement (a). Express your answer in degrees. Find the tension Tb in the cable in the arrangement (b). Express your answer in terms of w. Find the magnitude of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in terms of w.arrow_forward
- (Figure 1)In each case let ww be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w. Find the direction of the force exerted on the strut by the pivot in the arrangement (b). Express your answer in degrees.arrow_forwardA 70.0 cm, uniform, 40.0 N shelf is supported horizontally by two vertical wires attached to the sloping ceiling (Figure 1). A very small 20.0 N tool is placed on the shelf midway between the points where the wires are attached to it. Find the tension in the left-hand wire. Express your answer with the appropriate units.arrow_forwardFind the total bind Mev. binding energy for 13 Carbon, 6C (atomic mass = 13.0033554)arrow_forward
- What is the 27 energy absorbed in this endothermic Auclear reaction 2] Al + 'n → 27 Mg + ! H? (The atom mass of "Al is 26.981539u. and that of 11 Mg is 26.984341u) MeVarrow_forwardWhat is the energy released in this nuclear reaction 1 F + "', H-1 O+ He? 19 19 16 (The atomic mass of 1F is 18.998403 u, and that of 20 is 15.9949154) MeV.arrow_forwardWhat is the energy released in this B+ nuclear reaction خالد 2½ Al w/ Mg + ie? (The atomic mass of 11 Al is 23.9999394 and that > of 12 Mg is 23.985041 u) MeV.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY