
(a)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
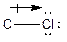
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
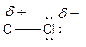
To find: Categorize all polar covalent bonds in the given compound (a)
(b)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
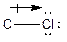
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
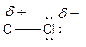
To find: Categorize all polar covalent bonds in the given compound (b)
(c)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
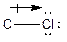
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
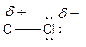
To find: Categorize all polar covalent bonds in the given compound (c)
(d)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
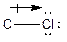
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
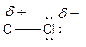
To find: Categorize all polar covalent bonds in the given compound (d)
(e)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
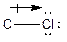
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
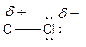
To find: Categorize all polar covalent bonds in the given compound (e)
(f)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
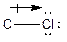
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
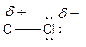
To find: Categorize all polar covalent bonds in the given compound (f)
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY (LL)-W/WILEYPLUS
- Consider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forwardHow many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forward
- Consider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forwardWhat kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forward
- Problem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forwardFeedback (4/10) 30% Retry Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the reactant and missing intermediates involved in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Incorrect, 6 attempts remaining :0: Draw the Reactant H H3CO H- HIO: Ö-CH3 CH3OH2* protonation H. a H (+) H Ο CH3OH2 O: H3C protonation CH3OH deprotonation > CH3OH nucleophilic addition H. HO 0:0 Draw Intermediate a Xarrow_forwardCan I please get the blank spaces answered/answers?arrow_forward
- 1. Identify the following alkenes as E or Z NH₂ Br 2. Draw the structures based on the IUPAC names (3R,4R)-3-bromo-4-fluoro- 1-hexene (Z)-4-bromo-2-iodo-3-ethyl- 3-heptene تر 3. For the following, predict all possible elimination product(s) and circle the major product. HO H₂SO4 Heat 80 F4 OH H2SO4 Heat 어요 F5 F6 1 A DII 4 F7 F8 F9 % & 5 6 7 * ∞ 8 BAB 3 E R T Y U 9 F D G H J K O A F11 F10arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





