
a)
Interpretation: The products of the following reactions are to be predicted.
Concept Introduction:
The cleavage of ether below acidic conditions results in the formation of two
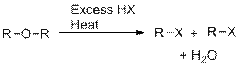
The cleavage of Phenyl ether below acidic conditions results in the formation of Phenol and Alkyl halide.
Phenol cannot be further used in the conversion of Halide, because either
Hydrogen iodide and Hydrogen bromide are used in the cleavage of Ethers. Hydrogen chloride is less significant and Hydrogen fluoride doesn’t cause acid cleavage of Ethers. The reactivity is a result of relative nucleophilicity of Halide ions.
b)
Interpretation: The products of the following reactions are to be predicted.
Concept Introduction:
The cleavage of ether below acidic conditions results in the formation of two Alkyl halides.
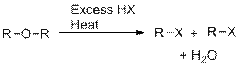
The cleavage of Phenyl ether below acidic conditions results in the formation of Phenol and Alkyl halide.
Phenol cannot be further used in the conversion of Halide, because either
Hydrogen iodide and Hydrogen bromide are used in the cleavage of Ethers. Hydrogen chloride is less significant and Hydrogen fluoride doesn’t cause acid cleavage of Ethers. The reactivity is a result of relative nucleophilicity of Halide ions.
c)
Interpretation: The products of the following reactions are to be predicted.
Concept Introduction:
The cleavage of ether below acidic conditions results in the formation of two Alkyl halides.
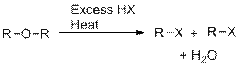
The cleavage of Phenyl ether below acidic conditions results in the formation of Phenol and Alkyl halide.
Phenol cannot be further used in the conversion of Halide, because either
Hydrogen iodide and Hydrogen bromide are used in the cleavage of Ethers. Hydrogen chloride is less significant and Hydrogen fluoride doesn’t cause acid cleavage of Ethers. The reactivity is a result of relative nucleophilicity of Halide ions.
d)
Interpretation: The products of the following reactions are to be predicted.
Concept Introduction:
The cleavage of ether below acidic conditions results in the formation of two Alkyl halides.
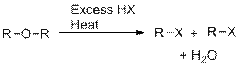
The cleavage of Phenyl ether below acidic conditions results in the formation of Phenol and Alkyl halide.
Phenol cannot be further used in the conversion of Halide, because either
Hydrogen iodide and Hydrogen bromide are used in the cleavage of Ethers. Hydrogen chloride is less significant and Hydrogen fluoride doesn’t cause acid cleavage of Ethers. The reactivity is a result of relative nucleophilicity of Halide ions.
e)
Interpretation: The products of the following reactions are to be predicted.
Concept Introduction:
The cleavage of ether below acidic conditions results in the formation of two Alkyl halides.
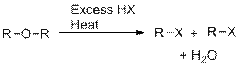
The cleavage of Phenyl ether below acidic conditions results in the formation of Phenol and Alkyl halide.
Phenol cannot be further used in the conversion of Halide, because either
Hydrogen iodide and Hydrogen bromide are used in the cleavage of Ethers. Hydrogen chloride is less significant and Hydrogen fluoride doesn’t cause acid cleavage of Ethers. The reactivity is a result of relative nucleophilicity of Halide ions.
f)
Interpretation: The products of the following reactions are to be predicted.
Concept Introduction:
The cleavage of ether below acidic conditions results in the formation of two Alkyl halides.
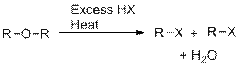
The cleavage of Phenyl ether below acidic conditions results in the formation of Phenol and Alkyl halide.
Phenol cannot be further used in the conversion of Halide, because either
Hydrogen iodide and Hydrogen bromide are used in the cleavage of Ethers. Hydrogen chloride is less significant and Hydrogen fluoride doesn’t cause acid cleavage of Ethers. The reactivity is a result of relative nucleophilicity of Halide ions.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
ORG.CHEM EBOOK W/BBWILEY PLUS>CUSTOM<
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward
- Write the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forwardClick the "draw structure" button to launch the drawing utility. Draw the structure of the alkene that yields the following set of oxidative cleavage products? draw structure ...arrow_forwardWrite the mechanism for the reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





