
a)
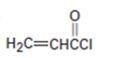
Interpretation:
Whether the compound shown will be a good dienophile or not is to be predicted.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. These reactions occur rapidly with dienophiles having electron withdrawing substituent groups in conjugation with the double bond.
To predict:
Whether the compound shown will be a good dienophile or not.
b)
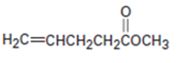
Interpretation:
Whether the compound shown will be a good dienophile or not is to be predicted.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. These reactions occur rapidly with dienophiles having electron withdrawing substituent groups in conjugation with the double bond.
To predict:
Whether the compound shown will be a good dienophile or not.
c)
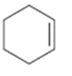
Interpretation:
Whether the compound shown will be a good dienophile or not is to be predicted.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. These reactions occur rapidly with dienophiles having electron withdrawing substituent groups in conjugation with the double bond.
To predict:
Whether the compound shown will be a good dienophile or not.
d)
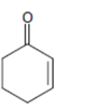
Interpretation:
Whether the compound shown will be a good dienophile or not is to be predicted.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. These reactions occur rapidly with dienophiles having electron withdrawing substituent groups in conjugation with the double bond.
To predict:
Whether the compound shown will be a good dienophile or not.
e)
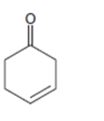
Interpretation:
Whether the compound shown will be a good dienophile or not is to be predicted.
Concept introduction:
In Diels-Alder reaction, a dienophile reacts with a diene to yield a cyclic adduct. The reaction takes place through 1,4 addition of the dienophile into the diene through a cyclic transition state. These reactions occur rapidly with dienophiles having electron withdrawing substituent groups in conjugation with the double bond.
To predict:
Whether the compound shown will be a good dienophile or not.
Trending nowThis is a popular solution!

Chapter 14 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
